Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
https://kanker-actueel.nl/NL/voordelen-van-ops-lidmaatschap-op-een-rijtje-gezet-inclusief-hoe-het-kookboek-en-de-recepten-op-basis-van-uitgangspunten-van-houtsmullerdieet-te-downloaden-enof-in-te-zien.html
8 maart 2016: Als aanvulling op onderstaand bericht hier enkele nieuwe studies met deze vorm van diagnose van beginnende slokdarmkanker. De abstracten plus referentielijsten staan onderaan artikel
Hier een algemene studie waarvan het volledige studierapport gratis is in te zien: Application of confocal laser endomicroscopy in the diagnosis and management of Barrett’s esophagus
en een studie met ratten waarvan het volledige studierapport ook gratis is in te zien, waarin er ook een stofje aan is toegevoegd die bepaalde receptoren activieert als het ware waardoor er nog meer is te zien, zie verderop in artikel foto van het verschil: Detection of fluorescent organic nanoparticles by confocal laser endomicroscopy in a rat model of Barrett's esophageal adenocarcinoma.
Je vraagt je toch af waarom dit soort opsporingstechnieken niet veel sneller gebruikt worden.
Het zou heel veel patienten behoeden voor belastende diagnose technieken en nog beter als er iets ontdekt wordt kan er veel sneller worden ingegrepen.
28 januari 2012: Bron: Mailonsunday
Engelse onderzoekers aan de Cambridge universiteit hebben een fluoriscerende keelspray ontwikkeld om slokdarmkanker vroeger op te sporen en beter te kunnen vaststellen waar de tumor zit en hoever deze is verspreid. De huidige methoden die worden gebruikt voor het opsporen en diagnosteren kunnen dermate onnauwkeurig zijn of te laat worden gedaan dat een deel van de patiënten een onnodige en te grote invasieve behandeling met inbegrip van verwijdering van hun slokdarm moeten ondergaan.
Nu hebben wetenschappers een fluoriscerende kleurstof spray ontwikkeld die zich hecht aan gezonde cellen in de slokdarm, maar die zich niet kan hechten aan kankercellen. Als deze spray wordt gebruikt geeft deze een duidelijke verwijzing naar de plaats waar de tumor zich ontwikkelt. Indien beginnende tumoren in deze fase worden gespot, kunnen de kankercellen worden verwijderd met een vorm van RFA - Radio Frequency Ablation.
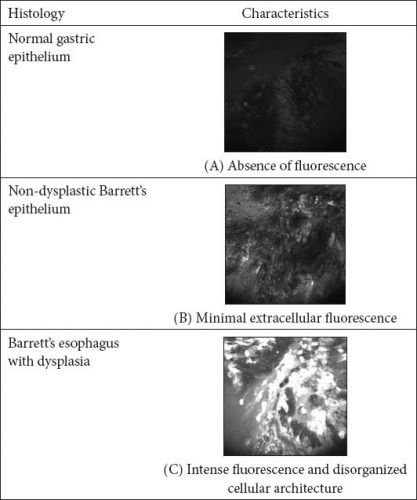
Foto: het verschil tussen scan zonder fluoriscerend stofje en met!!!!
Hoofdonderzoeker dr. Rebecca Fitzgerald zegt: "De huidige methoden voor het screenen op slokdarmkanker zijn controversieel - ze zijn duur, ongemakkelijk voor de patiënt en zijn niet volledig accuraat. Onze techniek belicht de exacte positie van een zich ontwikkelende slokdarmkanker en hoe ver deze is verspreid. En geeft een nauwkeuriger beeld dan andere technieken.
Met deze methode hebben de onderzoekers al kunnen voorkomen bij enkele patiënten uit de studiegroep dat deze een ingrijpende operatie zouden moeten ondergaan.
De onderzoekers testten deze behandeling op 80 biopten van mensen met de ziekte van Barrett (reflux), een aandoening die het risico op slokdarmkanker verhoogd. En zij testten de methode bij vier patiënten met slokdarmkanker.
De onderzoekers zeggen dat de gebruikte kleurstof relatief goedkoop is en waarschijnlijk weinig bijwerkingen kan veroorzaken omdat het gebruik maakt van een soort van tarwekiemen eiwit dat voorkomt in onze normale voeding.
Deze kleurstof bindt aan glycanen - suiker-moleculen - op het oppervlak van cellen in de slokdarm en de onderzoekers voegen een fluorescerend label eraan toe om het groen op te laten lichten onder het licht van een specifieke golflengte.
Het geheel kan dan bekeken worden met behulp van een endoscoop.
De glycanen tonen precies aan waar al kleine veranderingen plaatsvinden in het weefsel. Veranderingen die met andere methoden nog niet worden getraceerd.
Voordat de spray kan worden gebruikt zullen er meerdere studies moeten worden gedaan bij grotere groepen patiënten, maar de onderzoekers verwachten dat binnen 5 jaar deze methode beschikbaar zal komen voor algemeen gebruik.
Klik hier als u de originele engelstalige tekst waarvan bovenstaande een vrije vertaling is wilt lezen.
There are several studies that have demonstrated reasonable diagnostic accuracy in patients undergoing surveillance for Barrett’s esophagus from tertiary academic medical centers.
Ann Gastroenterol. 2014; 27(3): 193–199.
Application of confocal laser endomicroscopy in the diagnosis and management of Barrett’s esophagus
This article has been
cited by other articles in PMC.
Abstract
Confocal laser endomicroscopy is an advanced endoscopic imaging modality that can be used for the diagnosis of early mucosal dysplasia in various gastrointestinal conditions. It provides histology-like images at 1000-fold magnification. The technology offers potential advantages in the diagnosis of Barrett’s esophagus and early esophageal cancer due to the low yield of the current practice of surveillance endoscopy with biopsies. Confocal laser endomicroscopy has the potential to eliminate the need for biopsy, establish diagnosis and facilitate application of endoscopic therapy during the time of actual endoscopy. There are several studies that have demonstrated reasonable diagnostic accuracy in patients undergoing surveillance for Barrett’s esophagus from tertiary academic medical centers. However, the application of confocal laser endomicroscopy in routine clinical endoscopy is still in the process of refinement. Its role in the diagnosis and treatment of Barrett’s-associated dysplasia will continue to evolve with improvement in technology, criteria for diagnosis and experience among endoscopists in interpreting confocal imaging.
References
1.
Chandra S, Gorospe EC, Leggett CL, Wang KK. Barrett's esophagus in 2012: updates in pathogenesis, treatment, and surveillance. Curr Gastroenterol Rep. 2013;15:322. [PMC free article] [PubMed]2.
Peters FP, Curvers WL, Rosmolen WD, et al. Surveillance history of endoscopically treated patients with early Barrett's neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis Esophagus. 2008;21:475–479. [PubMed]3.
Dunbar KB, Okolo P, 3rd, Montgomery E, Canto MI. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645–654. [PMC free article] [PubMed]4.
De Palma GD. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770–5775. [PMC free article] [PubMed]5.
Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548–552. [PubMed]6.
O’Neil RG, Wu L, Mullani N. Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol Imaging Biol. 2005;7:388–392. [PubMed]7. Gorospe EC, Tian JM, Anderson M, et al. A Potential new marker of dysplasia: 2-Nbdg in Barrett's esophagus cell lines. Gastroenterology. 2011;140:S215–S215.
8.
Thekkek N, Maru DM, Polydorides AD, Bhutani MS, Anandasabapathy S, Richards-Kortum R. Pre-clinical evaluation of fluorescent deoxyglucose as a topical contrast agent for the detection of Barrett's-associated neoplasia during confocal imaging. Technol Cancer Res Treat. 2011;10:431–441. [PubMed]9.
Kato H, Takita J, Miyazaki T, et al. Glut-1 glucose transporter expression in esophageal squamous cell carcinoma is associated with tumor aggressiveness. Anticancer Res. 2002;22:2635–2639. [PubMed]10. Leggett CL, Sun G, Chowdhury S, et al. Topical esophageal delivery of a fluorescent marker of dysplasia in Barrett's esophagus: a feasibility study. Gastroenterology. 2013;144:S694–S694.
11.
Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. [PubMed]12.
Canto MI, Anandasabapathy S, Brugge W, et al. In vivo endomicroscopy improves detection of Barrett's esophagus-related neoplasia: a multicenter international randomized controlled trial (with video) Gastrointest Endosc. 2014;79:211–221. [PMC free article] [PubMed]13.
Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 2011;43:882–891. [PubMed]14.
Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett's esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19–24. [PMC free article] [PubMed]15.
Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465–472. [PMC free article] [PubMed]16.
Gaddam S, Mathur SC, Singh M, et al. Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrett's esophagus. Am J Gastroenterol. 2011;106:1961–1969. [PubMed]17.
Gorospe EC, Leggett CL, Sun G, et al. Diagnostic performance of two confocal endomicroscopy systems in detecting Barrett's dysplasia: a pilot study using a novel bioprobe in ex vivo tissue. Gastrointest Endosc. 2012;76:933–938. [PubMed]18.
Johnson EA, De Lee R, Agni R, Pfau P, Reichelderfer M, Gopal DV. Probe-based confocal laser endomicroscopy to guide real-time endoscopic therapy in Barrett's esophagus with dysplasia. Case Rep Gastroenterol. 2012;6:285–292. [PMC free article] [PubMed]19.
Konda VJ, Chennat JS, Hart J, Waxman I. Confocal laser endomicroscopy: potential in the management of Barrett's esophagus. Dis Esophagus. 2010;23:E21–E31. [PubMed]20.
Leung KK, Maru D, Abraham S, Hofstetter WL, Mehran R, Anandasabapathy S. Optical EMR: confocal endomicroscopy-targeted EMR of focal high-grade dysplasia in Barrett's esophagus. Gastrointest Endosc. 2009;69:170–172. [PubMed]21.
Li Z, Yu T, Zuo XL, et al. Confocal laser endomicroscopy for in vivo diagnosis of gastric intraepithelial neoplasia: a feasibility study. Gastrointest Endosc. 2010;72:1146–1153. [PubMed]22.
Wallace MB, Crook JE, Saunders M, et al. Multicenter, randomized, controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett's esophagus. Gastrointest Endosc. 2012;76:539–547. e531. [PubMed]23.
Pohl H, Rosch T, Vieth M, et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's oesophagus. Gut. 2008;57:1648–1653. [PubMed]24.
Bajbouj M, Vieth M, Rosch T, et al. Probe-based confocal laser endomicroscopy compared with standard four-quadrant biopsy for evaluation of neoplasia in Barrett's esophagus. Endoscopy. 2010;42:435–440. [PubMed]25.
Gupta A, Attar BM, Koduru P, Murali AR, Go BT, Agarwal R. Utility of confocal laser endomicroscopy in identifying high-grade dysplasia and adenocarcinoma in Barrett's esophagus: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:369–377. [PubMed]26.
Li M, Anastassiades CP, Joshi B, et al. Affinity peptide for targeted detection of dysplasia in Barrett's esophagus. Gastroenterology. 2010;139:1472–1480. [PMC free article] [PubMed]27.
Jayasekera C, Taylor AC, Desmond PV, Macrae F, Williams R. Added value of narrow band imaging and confocal laser endomicroscopy in detecting Barrett's esophagus neoplasia. Endoscopy. 2012;44:1089–1095. [PubMed]28.
Verna C, Feyles E, Lorenzi L, et al. I-SCAN targeted versus random biopsies in Barrett's oesophagus. Dig Liver Dis. 2014;46:131–134. [PubMed]29.
Yun SH, Tearney GJ, Vakoc BJ, et al. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006;12:1429–1433. [PMC free article] [PubMed]
the combination of NP platform and confocal laser endomicroscopy could play an important role for highlighting esophageal cancer conditions. This result supports the potential of this strategy as a targeted carrier for photoactive and bioactive molecules in esophageal cancer diagnosis and treatment.
Int J Nanomedicine. 2015; 10: 6811–6823.
Detection of fluorescent organic nanoparticles by confocal laser endomicroscopy in a rat model of Barrett’s esophageal adenocarcinoma
Elisa Dassie,
1,2,* Diletta Arcidiacono,
2,3,* Iga Wasiak,
4 Nunzio Damiano,
5 Luigi Dall’Olmo,
6 Cinzia Giacometti,
7 Sonia Facchin,
3 Mauro Cassaro,
7 Ennio Guido,
8 Franca De Lazzari,
8 Oriano Marin,
9,10 Tomasz Ciach,
4 Suzanne Fery-Forgues,
11,12 Alfredo Alberti,
1,2 Giorgio Battaglia,
13 and
Stefano Realdon13
Abstract
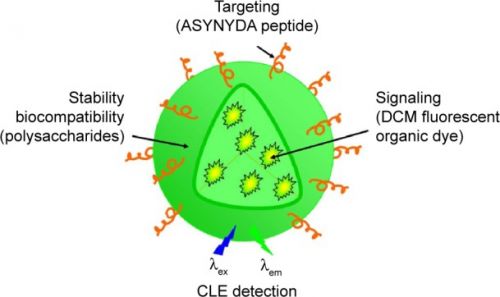
For many years, novel strategies for cancer detection and treatment using nanoparticles (NPs) have been developed. Esophageal adenocarcinoma is the sixth leading cause of cancer-related deaths in Western countries, and despite recent advances in early detection and treatment, its prognosis is still very poor. This study investigated the use of fluorescent organic NPs as potential diagnostic tool in an experimental in vivo model of Barrett’s esophageal adenocarcinoma. NPs were made of modified polysaccharides loaded with [4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran] (DCM), a well-known fluorescent dye. The NP periphery might or might not be decorated with ASYNYDA peptide that has an affinity for esophageal cancer cells. Non-operated and operated rats in which gastroesophageal reflux was surgically induced received both types of NPs (NP-DCM and NP-DCM-ASYNYDA) by intravenous route. Localization of mucosal NPs was assessed in vivo by confocal laser endomicroscopy, a technique which enables a “real time” and in situ visualization of the tissue at a cellular level. After injection of NP-DCM and NP-DCM-ASYNYDA, fluorescence was observed in rats affected by esophageal cancer, whereas no signal was observed in control non-operated rats, or in rats with simple esophagitis or Barrett’s esophagus mucosa. Fluorescence was observable in vivo 30 minutes after the administration of NPs. Interestingly, NP-DCM-ASYNYDA induced strong fluorescence intensity 24 hours after administration. These observations suggested that NPs could reach the tumor cells, likely by enhanced permeability and retention effect, and the peptide ASYNYDA gave them high specificity for esophageal cancer cells. Thus, the combination of NP platform and confocal laser endomicroscopy could play an important role for highlighting esophageal cancer conditions. This result supports the potential of this strategy as a targeted carrier for photoactive and bioactive molecules in esophageal cancer diagnosis and treatment.
References
1.
Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–146. [PubMed]2.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [PubMed]3.
Bennett M, Mashimo H. Molecular markers and imaging tools to identify malignant potential in Barrett’s esophagus. World J Gastrointest Pathophysiol. 2014;5(4):438–449. [PMC free article] [PubMed]4.
Phillips WA, Lord RV, Nancarrow DJ, Watson DI, Whiteman DC. Barrett’s esophagus. J Gastroenterol Hepatol. 2011;26(4):639–648. [PubMed]5.
Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122(1):26–33. [PubMed]6.
Zhang WJ, Sui YX, Budha A, et al. Affinity peptide developed by phage display selection for targeting gastric cancer. World J Gastroenterol. 2012;18(17):2053–2060. [PMC free article] [PubMed]7.
Xu D, Wu F, Chen Y, Wei L, Yuan W. pH-sensitive degradable nanoparticles for highly efficient intracellular delivery of exogenous protein. Int J Nanomedicine. 2013;8(1):3405–3414. [PMC free article] [PubMed]8.
Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21(5):797–802. [PubMed]9.
Bigini P, Previdi S, Casarin E, et al. In vivo fate of avidin-nucleic acid nano-assemblies as multifunctional diagnostic tools. ACS Nano. 2014;8(1):175–187. [PubMed]10.
Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed]11.
Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010;138(3):828–833. [PMC free article] [PubMed]12.
Johnson EA, De Lee R, Agni R, Pfau P, Reichelderfer M, Gopal DV. Probe-based confocal laser endomicroscopy to guide real-time endoscopic therapy in Barrett’s esophagus with dysplasia. Case Rep Gastroenterol. 2012;6(2):285–292. [PMC free article] [PubMed]13.
Goetz M, Kiesslich R. Confocal endomicroscopy: in vivo diagnosis of neoplastic lesions of the gastrointestinal tract. Anticancer Res. 2008;28(1B):353–360. [PubMed]14.
Bertani H, Pigò F, Dabizzi E, et al. Advances in endoscopic visualization of Barrett’s esophagus: the role of confocal laser endomicroscopy. Gastroentero Res Pract. 2012;2012:493961. [PMC free article] [PubMed]15.
Buda A, Facchin S, Dassie E, et al. Detection of a fluorescent-labeled avidin-nucleic acid nanoassembly by confocal laser endomicroscopy in the microvasculature of chronically inflamed intestinal mucosa. Int J Nanomedicine. 2015;10:399–408. [PMC free article] [PubMed]16.
Buda A, Hatem G, Neumann H, et al. Confocal laser endomicroscopy for prediction of disease relapse in ulcerative colitis: a pilot study. J Crohns Colitis. 2014;8(4):304–311. [PubMed]17.
Wang R, Xiao R, Zeng Z, Xu L, Wang J. Application of poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) block copolymers and their derivatives as nanomaterials in drug delivery. Int J Nanomedicine. 2012;7:4185–4198. [PMC free article] [PubMed]18.
Shin SJ, Beech JR, Kelly KA. Targeted nanoparticles in imaging: paving the way for personalized medicine in the battle against cancer. Integr Biol (Camb) 2013;5(1):29–42. [PubMed]19.
Sturm MB, Joshi BP, Lu S, et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci Transl Med. 2013;5(184):184ra61. [PMC free article] [PubMed]20.
Sturm MB, Piraka C, Elmunzer BJ, et al. In vivo molecular imaging of Barrett’s esophagus with confocal laser endomicroscopy. Gastroenterology. 2013;145(1):56–58. [PMC free article] [PubMed]21.
Raggi M, Langer R, Feith M, Friess H, Schauer M, Theisen J. Successful evaluation of a new animal model using mice for esophageal adenocarcinoma. Langenbecks Arch Surg. 2010;395(4):347–350. [PubMed]22.
Garman KS, Orlando RC, Chen X. Review: experimental models for Barrett’s esophagus and esophageal adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1231–G1243. [PMC free article] [PubMed]23.
Ingravallo G, Dall’Olmo L, Segat D, et al. CDX2 hox gene product in a rat model of esophageal cancer. J Exp Clin Cancer Res. 2009;28(108):1–6. [PMC free article] [PubMed]24.
Oh DS, DeMeester SR, Dunst CM, et al. Validation of a rodent model of Barrett’s esophagus using quantitative gene expression profiling. Surg Endosc. 2009;23(6):1346–1352. [PubMed]25.
Realdon S, Dassie E, Fassan M, et al. In vivo molecular imaging of HER2 expression in a rat model of Barrett’s esophagus adenocarcinoma. Dis Esophagus. 2015;28(4):394–403. [PubMed]26.
Dall’Olmo L, Fassan M, Dassie E, et al. Role of proton pump inhibitor on esophageal carcinogenesis and pancreatic acinar cell metaplasia development: an experimental in vivo study. PLoS One. 2014;9(11):e112862. [PMC free article] [PubMed]27.
Muangsiri W, Kirsch LE. The protein-binding and drug release properties of macromolecular conjugates containing daptomycin and dextran. Int J Pharm. 2006;315(1–2):30–43. [PubMed]28.
Fuentes M, Segura RL, Abian O, et al. Determination of protein-protein interactions through aldehyde-dextran intermolecular cross-linking. Proteomics. 2004;4(9):2602–2607. [PubMed]29.
Field GB, Noble RL. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990;35(3):161–214. [PubMed]30.
Carpino LA, Henklein P, Foxman BM, et al. The solid state and solution structure of HAPyU. J Org Chem. 2001;66(15):5245–5247. [PubMed]31.
Khemakhem K, Soulié M, Brousses R, Ammar H, Abid S, Fery-Forgues S. Small iminocoumarin derivatives as red emitters: from biological imaging to highly photoluminescent non-doped micro- and nanofibres. Chem Eur J. 2015;21(21):7927–7937. [PubMed]32.
Lu S, Lowe AW, Triadafilopoulos G, et al. Endoscopic evaluation of esophago-gastro-jejunostomy in rat model of Barrett’s esophagus. Dis Esophagus. 2009;22(4):323–330. [PMC free article] [PubMed]33. Ciach T, Wasiak I. Patent WO 2013/137755 A1 Process for the preparation of polysaccharide nanoparticles.
34.
Lisman A, Butruk B, Wasiak I, Ciach T. Dextran/Albumin hydrogel sealant for Dacron (R) vascular prosthesis. J Biomater Appl. 2014;28(9):1386–1396. [PubMed]35.
Rurack K, Spieles M. Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600–1,000 nm. Anal Chem. 2011;83(4):1232–1242. [PubMed]36.
Yan D, Fan G, Guan Y, Meng Q, Li C, Wang J. Tuning solid-state blue and red luminescence by the formation of solvate crystals. Phys Chem Chem Phys. 2013;15(45):19845–19852. [PubMed]37.
Li Y, Gobin AM, Dryden GW, et al. Infrared light-absorbing gold/gold sulfide nanoparticles induce cell death in esophageal adenocarcinoma. Int J Nanomedicine. 2013;8(1):2153–2161. [PMC free article] [PubMed]38.
Vieth M, Ell C, Gossner L, May A, Stolte M. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy. 2004;36(9):776–781. [PubMed]
slokdarmkanker, fluoriscerende spray, diagnostiek, ziekte van Barrett
Gerelateerde artikelen





Plaats een reactie ...
Reageer op "Diagnose: Slokdarmkanker zou sneller en accurater op zijn te sporen via een fluorescerende spray en soms kunnen daarmee belastende operaties voorkomen worden"