Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
https://kanker-actueel.nl/NL/voordelen-van-ops-lidmaatschap-op-een-rijtje-gezet-inclusief-hoe-het-kookboek-en-de-recepten-op-basis-van-uitgangspunten-van-houtsmullerdieet-te-downloaden-enof-in-te-zien.html
4 november 2015: Bron: PLoS One. 2013; 8(5): e63682. Published online 2013 May 28. doi: 10.1371/journal.pone.0063682
Het verwijderen van een hooggradige hersentumor - glioma blastoma, met hulp van 5-aminolevulinic acid (5-ALA) dat de tumorcellen doet oplichten geeft betere resultaten op volledige verwijdering van het tumorweefsel, langere ziektevrije tijd en betere overall overleving in vergelijking met een conventionele operatie waarbij gebruik wordt gemaakt van MRI begeleiding en open schedel operatie. Dit blijkt uit een grote reviewstudie van gerandomiseerde en prospectieve studies.
Uit alle studies samen blijkt opereren met 5-aminolevulinic acid (5-ALA) statistisch significant betere resultaten te geven op zowel meer verwijdering van tumorweefsel, ziektevrije tijd en mediane overall overleving. Dit gaat niet op voor laaggradige hersentumoren omdat deze niet oplichten wegens gebrek aan stofje dat tumorcellen doet oplichten.
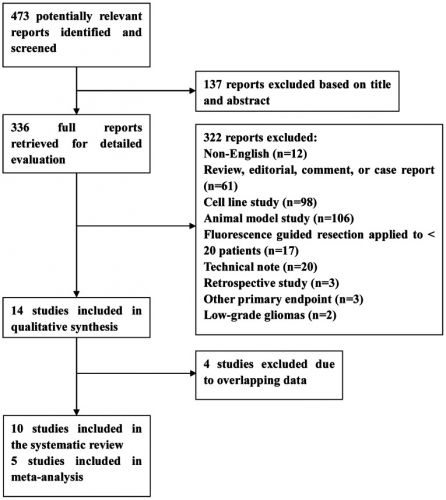
Hier de hoeveelheid studies die meegenomen zijn in de meta analyse:
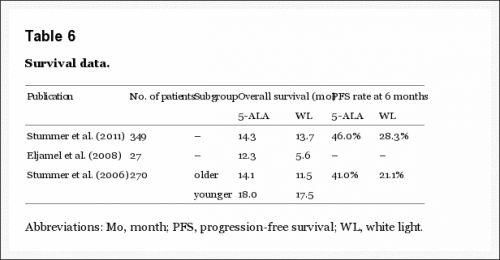
De overleving uit enkele studies:
Based on available literature, there is level 2 evidence that 5-ALA-guided surgery is more effective than conventional neuronavigation-guided surgery in increasing diagnostic accuracy and extent of tumor resection, enhancing quality of life, or prolonging survival in patients with high-grade malignant gliomas.
PLoS One. 2013; 8(5): e63682.
Intraoperative Fluorescence-Guided Resection of High-Grade Malignant Gliomas Using 5-Aminolevulinic Acid–Induced Porphyrins: A Systematic Review and Meta-Analysis of Prospective Studies
Shiguang Zhao,
#1,2,* Jianing Wu,
#1,2 Chunlei Wang,
1,2 Huailei Liu,
1,2 Xingli Dong,
3 Chen Shi,
4 Changbin Shi,
5 Yaohua Liu,
1,2 Lei Teng,
1,2 Dayong Han,
1,2 Xiaofeng Chen,
1,2 Guang Yang,
1,2 Ligang Wang,
1,2 Chen Shen,
1,2 and
Huadong Li1,2
Jonathan A. Coles, Editor
This article has been
cited by other articles in PMC.
Abstract
Background
We performed a systematic review and meta-analysis to address the (added) value of intraoperative 5-aminolevulinic acid (5-ALA)-guided resection of high-grade malignant gliomas compared with conventional neuronavigation-guided resection, with respect to diagnostic accuracy, extent of tumor resection, safety, and survival.
Methods and Findings
An electronic database search of Medline, Embase, and the Cochrane Library was undertaken. The review process followed the guidelines of the Cochrane Collaboration. 10 studies matched all selection criteria, and were thus used for qualitative synthesis. 5-ALA-guided resection demonstrated an overall sensitivity of 0.87 (95% confidence interval , 0.81–0.92), specificity of 0.89 (95% CI, 0.79–0.94), positive likelihood ratio (LR) of 7.62 (95% CI, 3.87–15.01), negative LR of 0.14 (95% CI, 0.09–0.23), and diagnostic odds ratio (OR) of 53.06 (95% CI, 18.70–150.51). Summary receiver operating characteristic curves (SROC) showed an area under curve (AUC) of 94%. Contrast-enhancing tumor was completely resected in patients assigned 5-ALA as compared with patients assigned white light. Patients in the 5-ALA group had higher 6-month progression free survival and overall survival than those in the white light group.
Conclusion
Based on available literature, there is level 2 evidence that 5-ALA-guided surgery is more effective than conventional neuronavigation-guided surgery in increasing diagnostic accuracy and extent of tumor resection, enhancing quality of life, or prolonging survival in patients with high-grade malignant gliomas.
References
1.
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, et al. (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95: 190–198 [PubMed]2.
Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, et al. (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20: 1375–1382 [PubMed]3. Bennett H, Godlee RJ (1884) Excision of a tumour from the brain. Lancet 2: 1090–1091
4.
Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, et al. (2008) 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly 138: 180–185 [PubMed]5.
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, et al. (2009) Independent association of extent of resection with survival in patients with malignant brain astro-cytoma. J Neurosurg 110: 156–162 [PubMed]6.
Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, et al. (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12: 1062–1070 [PubMed]7.
Regula J, MacRobert AJ, Gorchein A, Buonaccorsi GA, Thorpe SM, et al. (1995) Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX–a pilot study. Gut 36: 67–75 [PMC free article] [PubMed]8.
Roberts DW, Valdes PA, Harris BT, Hartov A, Fan X, et al. (2012) Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic Acid). Neurosurg Clin N Am 23: 371–377 [PMC free article] [PubMed]9.
Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, et al. (1998) Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir 140: 995–1000 [PubMed]10.
Duffner F, Ritz R, Freudenstein D, Weller M, Dietz K, et al. (2005) Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J Neurooncol 71: 107–111 [PubMed]11.
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, et al. (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7: 392–401 [PubMed]12.
Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, et al. (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J Neurosurg 114: 613–623 [PubMed]13.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100. [PMC free article] [PubMed]14.
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3: 25. [PMC free article] [PubMed]15.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97–109 [PMC free article] [PubMed]16.
Walker E, Hernandez AV, Kattan MW (2008) Meta-analysis: Its strengths and limitations. Cleve Clin J Med 75: 431–439 [PubMed]17.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560 [PMC free article] [PubMed]18.
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634 [PMC free article] [PubMed]19.
Panciani PP, Fontanella M, Schatlo B, Garbossa D, Agnoletti A, et al. (2012) Fluorescence and image guided resection in high grade glioma. Clin Neurol Neurosurg 114: 37–41 [PubMed]20.
Stummer W, Nestler U, Stockhammer F, Krex D, Kern BC, et al. (2011) Favorable outcome in the elderly cohort treated by concomitant temozolomide radiochemotherapy in a multicentric phase II safety study of 5-ALA. J Neurooncol 103: 361–370 [PubMed]21.
Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, et al. (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J Neurosurg 114: 595–603 [PMC free article] [PubMed]22.
Ewelt C, Floeth FW, Felsberg J, Steiger HJ, Sabel M, et al. (2011) Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg 113: 541–547 [PubMed]23.
Diez Valle R, Tejada Solis S, Idoate Gastearena MA, García de Eulate R, Domínguez Echávarri P, et al. (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol 102: 105–113 [PubMed]24.
Eljamel MS (2009) Which intracranial lesions would be suitable for 5-aminolevulenic acid-induced fluorescence-guided identification, localization, or resection? A prospective study of 114 consecutive intracranial lesions. Clin Neurosurg 56: 93–97 [PubMed]25.
Eljamel MS, Goodman C, Moseley H (2008) ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci 23: 361–367 [PubMed]26.
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, et al. (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93: 1003–13 [PubMed]27.
Valdes PA, Kim A, Brantsch M, Niu C, Moses ZB, et al. (2011) delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol 13: 846–856 [PMC free article] [PubMed]28.
Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, et al. . (2007) Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo) 47: 210–213; discussion 213–214. [PubMed]29.
Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, et al. (2011) Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg 76: 120–127 [PubMed]30.
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, et al. . (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62: 564–576; discussion 564–576. [PubMed]31.
Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, et al. (2012) Gross Total Resection Rates in Contemporary Glioblastoma Surgery: Results of an Institutional Protocol Combining 5-ALA Intraoperative Fluorescence Imaging and Brain Mapping. Neurosurgery 71: 927–936 [PubMed]32.
Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, et al. . (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery 65: 1070–1076; discussion 1076–1077. [PubMed]33.
Panciani PP, Fontanella M, Garbossa D, Agnoletti A, Ducati A, et al. (2012) 5-aminolevulinic acid and neuronavigation in high-grade glioma surgery: results of a combined approach. Neurocirugia (Astur) 23: 23–28 [PubMed]34.
Idoate MA, Díez Valle R, Echeveste J, Tejada S (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31: 575–582 [PubMed]35.
Floeth FW, Sabel M, Ewelt C, Stummer W, Felsberg J, et al. (2011) Comparison of (18) F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging 38: 731–741 [PubMed]36.
Pichlmeier U, Bink A, Schackert G, Stummer W (2008) Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 10: 1025–1034 [PMC free article] [PubMed]37.
Stepp H, Beck T, Pongratz T, Meinel T, Kreth FW, et al. (2007) ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol 26: 157–164 [PubMed]38.
Teng L, Nakada M, Zhao SG, Endo Y, Furuyama N, et al. (2011) Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer 104: 798–807 [PMC free article] [PubMed]39.
Zhao SG, Chen XF, Wang LG, Yang G, Han DY, et al. . (2012) Increased Expression of ABCB6 Enhances Protoporphyrin IX Accumulation and Photodynamic Effect in Human Glioma. Ann Surg Oncol. In press. [PubMed]40.
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection ofmalignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34: 45–61 [PubMed]41.
Sanai N, Berger MS. (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62: 753–764;discussion 264–266. [PubMed]42.
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277–1280 [PubMed]43.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, et al. (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28: 1963–1972 [PubMed]44.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [PubMed]
glioblastoom, studies, hersentumoren, kytogeendieet, 5-aminolevulinic acid (5-ALA), PDT, operatie
Gerelateerde artikelen





Met vriendelijke groeten, kees