Uit een tussenevaluatie van een kleine gerandomiseerde studie (30 patiënten totaal) blijkt dat wanneer patiénten met gevorderde nierkanker naast de immuuntherapie met ipilimumab plus nivolumab tijdens de infusen met nivolumab ook oraal een probioticum genaamd Clostridium butyricum CBM 588 innamen de objectieve respons op de immuuntherapie sterk verbeterde met 38 procent. Ook zagen de onderzoekers in een tussenevaluatie na 12,5 maanden een beduidend langere progressievrije overleving (mediaan 55,0 weken vs 10,7 weken) en een trend naar een betere algehele overleving (mediane overleving was echter nog niet bereikt in beide groepen bij de tussenanalyse)
In onderstaande grafiek de resultaten in een tussenevaluatie 15 april 2021 en 28 februari 2022 gepubliceerd in Nature:
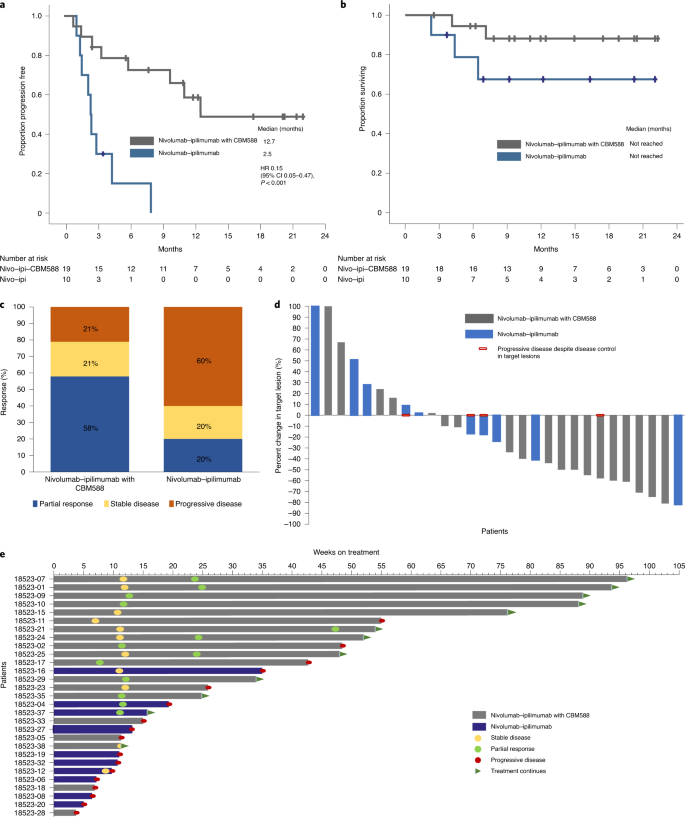
a,b, Progression-free response (a) and overall survival (b). c–e, Best response by treatment arm (c), best change in target lesions from baseline (d), and a swimmers plot showing the response and survival characteristics (e). The data are from n = 29 patients (19 patients in the nivolumab–ipilimumab with CBM588 arm and 10 patients in the nivolumab–ipilimumab arm). The Kaplan–Meier log-rank test was used to compare survival between the two arms.
De studie opzet:
Patiënten met ten minste op twee plaatsen uitgezaaide voorbehandelde nierkanker werden willekeurig toegewezen aan vier cycli van nivolumab plus ipilimumab therapie om de 3 weken, gevolgd door maandelijks onderhoud met nivolumab, gegeven met of zonder CBM-588 als een oraal supplement van 80 mg tweemaal daags.
Het primaire einddoel van deze studie was een verandering in verschillende soorten Bifidobacterium.
Na 12 weken behandeling werd geëvalueerd bij 19 patiënten die CBM-588 kregen en bij 10 controles.
Gepaarde ontlastingsmonsters toonden aan dat patiënten die CBM-588 kregen een verandering in hun microbioomsamenstelling hadden, met niet-significante toenames in Bifidobacterium, Bifidobacteriumes en Bifidobacteriaceae.
Daarentegen hadden patiënten die nivolumab plus ipilimumab kregen zonder CBM-588 een afname bij alle drie de bacteriestammen en, in tegenstelling tot hun tegenhangers die CBM-588 kregen, testten ze niet positief op C. butyricum.
Het gebruik van CBM-588 was ook geassocieerd met een hogere objectieve respons (58 vs. 20%), evenals een significant langere progressievrije overleving (mediaan 55,0 vs 10,7 weken) en een trend naar een betere algehele overleving (mediaan niet bereikt in beide armen). ).
De frequenties van graad 3 en ernstigere bijwerkingen waren "vergelijkbaar" in de twee groepen, hoewel Dr. Nazli Dizman de woordvoerder van de studiepresentatie waarschuwde dat één patiënt die CBM-588 kreeg graad 4 neutropenie ontwikkelde.
"Beperkingen van deze studie zijn onder meer de kleine omvang" en dat het op één enkele locatie werd uitgevoerd, zei Meza, eraan toevoegend dat de "resultaten niettemin intrigerend zijn en verder onderzoek rechtvaardigen."
Het volledige studierapprot is gratis in te zien of te downloaden. Klik daarvoor op de titel van het abstract:
- Article
- Open Access
- Published:
Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial
Nature Medicine (2022)
Abstract
Previous studies have suggested that the gut microbiome influences the response to checkpoint inhibitors (CPIs) in patients with cancer. CBM588 is a bifidogenic live bacterial product that we postulated could augment CPI response through modulation of the gut microbiome. In this open-label, single-center study (NCT03829111), 30 treatment-naive patients with metastatic renal cell carcinoma with clear cell and/or sarcomatoid histology and intermediate- or poor-risk disease were randomized 2:1 to receive nivolumab and ipilimumab with or without daily oral CBM588, respectively. Stool metagenomic sequencing was performed at multiple timepoints. The primary endpoint to compare the relative abundance of Bifidobacterium spp. at baseline and at 12 weeks was not met, and no significant differences in Bifidobacterium spp. or Shannon index associated with the addition of CBM588 to nivolumab–ipilimumab were detected. Secondary endpoints included response rate, progression-free survival (PFS) and toxicity. PFS was significantly longer in patients receiving nivolumab–ipilimumab with CBM588 than without (12.7 months versus 2.5 months, hazard ratio 0.15, 95% confidence interval 0.05–0.47, P = 0.001). Although not statistically significant, the response rate was also higher in patients receiving CBM588 (58% versus 20%, P = 0.06). No significant difference in toxicity was observed between the study arms. The data suggest that CBM588 appears to enhance the clinical outcome in patients with metastatic renal cell carcinoma treated with nivolumab–ipilimumab. Larger studies are warranted to confirm this clinical observation and elucidate the mechanism of action and the effects on microbiome and immune compartments.
References
-
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
-
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
-
Salgia, N. J. et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur. Urol. 78, 498–502 (2020).
-
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
-
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
-
Motzer, R. J. et al. NCCN guidelines insights: kidney cancer, version 2.2020. J. Natl Compr. Canc. Netw. 17, 1278–1285 (2019).
-
Escudier, B. et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30, 706–720 (2019).
-
Choueiri, T. K. et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial. Ann. Oncol. 31 (Suppl. 4), S1142–S1215 (2020).
-
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
-
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
-
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
-
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 20, 1370–1385 (2019).
-
Hagihara, M. et al. Clostridium butyricum modulates the microbiome to protect intestinal barrier function in mice with antibiotic-induced dysbiosis. iScience 23, 100772 (2020).
-
Ariyoshi, T. et al. Clostridium butyricum MIYAIRI 588-induced protectin D1 has an anti-inflammatory effect on antibiotic-induced intestinal disorder. Front. Microbiol. 11, 587725 (2020).
-
Isa, K. et al. Safety assessment of the Clostridium butyricum MIYAIRI 588® probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Hum. Exp. Toxicol. 35, 818–832 (2016).
-
Tomita, Y. et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol. Res. 8, 1236–1242 (2020).
-
Derosa, L. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444 (2018).
-
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
-
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
-
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109 (2015).
-
Rossen, N. G. et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149, 110–118 (2015).
-
DeFilipp, Z. et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant.N. Engl. J. Med. 381, 2043–2050 (2019).
-
Francisco-Anderson, L. et al. Abstract PS11-27: a phase I/II clinical trial of EDP1503 with pembrolizumab for triple-negative breast cancer. Cancer Res. 81 (4 Suppl.), abstr. PS11-27 (2021).
-
Adams, S. et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 397–404 (2019).
-
Dudani, S. et al. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw. Open 4, e2021869 (2021).
-
Reichardt, N. et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 12, 610–622 (2018).
-
Louis, P. & Flint, H. J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41 (2017).
-
Okamoto, T. et al. Preventive efficacy of butyrate enemas and oral administration of Clostridium butyricum M588 in dextran sodium sulfate-induced colitis in rats. J. Gastroenterol. 35, 341–346 (2000).
-
Takahashi, M. et al. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 41, 219–226 (2004).
-
Grasso, C. S. et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 38, 500–515 (2020).
-
Cremonesi, E. et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut 67, 1984–1994 (2018).
-
Choueiri, T. K. et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin. Cancer Res. 22, 5461–5471 (2016).
-
Dizman, N. et al. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. 10, 79–86 (2021).
-
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
-
Derosa, L. et al. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 11, 2396–2412 (2021).
-
Zhang, X. et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 70, 761–774 (2021).
-
Busi, S. B., Leon, K. B. D., Wall, J. D. & Amos-Landgraf, J. M. Abstract 4987: biofilm-producing sulfate-reducing bacteria suppress tumor burden in a rat model of colon cancer. Cancer Res. 78 (13 Suppl.), abstr. 4987 (2018).
-
Liu, C. M. et al. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol. 12, 56 (2012).
-
Liu, C. M. et al. FungiQuant: a broad-coverage fungal quantitative real-time PCR assay. BMC Microbiol. 12, 255 (2012).
-
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
-
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
-
Lu, J. et al. Bracken: estimating species abundance in metagenomics data. PeerJ Comput. Sci. 3, e104 (2017).
-
Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 10, e65088 (2021).
-
Chalmin, F. et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471 (2010).
Acknowledgements
We thank the patients and families for their participation in this study. Funding for the study was provided by a grant from the Gateway for Cancer Research (S.K.P; Grant ID: G-20-100). CBM588 was supplied by Miyarisan Pharmaceuticals Co., Ltd. and OSEL, Inc.
Author information
Affiliations
Contributions
Study conception and design: N.S., P.B., P.F., J.H., S.K.H., S.K.P. Project supervision: N.S., P.B., T.D., J.T., M.T., M.K., S.K.H., S.K.P. Participant recruitment and coordination: N.D., L.M., P.B., V.M., M.L., J.H., Z.Z., N.S., S.S., J.M., N.C., A.C.-R., R.M., S.K.P. Data collection and processing: N.D., L.M., P.B., M.A., V.M., M.L., J.H., J.G., L.R., M.T., K.O., S.H., S.K.H., S.K.P. Clinical data analysis: N.D., P.B., P.F., Y.C., J.H., S.K.P. Microbiome analysis: N.D., P.F., Y.C., J.G., M.T., K.O., S.H., S.K.H., S.K.P. Cytokine analysis: N.D., M.A., P.F., Y.C., M.K., S.K.P. Manuscript preparation: N.D., L.M., P.B., M.A., P.F., Y.C., J.H., M.T., K.O., S.H., M.K., S.K.H., S.K.P. Manuscript review and editing: all co-authors.
Corresponding authors
Gerelateerde artikelen
- Poeptransplantatie vergroot effectiviteit van immuuntherapie en vermindert ernstige bijwerkingen bij uitgezaaide gevorderde niercelkanker copy 1
- Specificiteit van het menselijk leukocyten antigeen (HLA) blijkt voorspellende biomarker voor effectiviteit van immuuntherapie met anti-PD medicijnen - checkpointremmers bij patienten met gevorderde niercelkanker
- Immuuntherapie met pembrolizumab geeft betere ziektevrije overleving (77 vs 68 procent) na operatie bij patiënten met nierkanker die een hoog risico liepen op recidief in vergelijking met een placebo
- Cabozantinib toegevoegd aan immuuntherapie met nivolumab en ipilimumab geeft betere ziekteprogressievrije tijd en ziektecontrole bij uitgezaaide onbehandelde nierkanker in vergelijking met placebo naast nivolumab en ipilimumab
- Immuuncheckpointremmers nivolumab + ipilimumab of pembrolizumab + axitinib geeft toch ziektecontrole en remissies in de klinische praktijk bij patiënten met gevorderde uitgezaaide nierkanker met een slechte prestatiestatus volgens ECOG PS ≥2
- Clostridium butyricum (CBM 588) een probioticum toegevoegd aan nivolumab plus ipilimumab verbeterd sterk de repons en progressievrije tijd voor patienten met uitgezaaide nierkanker
- Immuuntherapie met avelumab plus VEGF-remmer axitinib vermindert kans op recidief en meer ziektevrije overleving bij nierkankerpatienten (stadium III) na operatie
- Combinatiebehandelingen met vormen van immuuntherapie en anti-PD medicijnen geven veelbelovende resultaten bij (gevorderde) nierkanker. Hier de belangrijkste studies van afgelopen jaar
- Immuuntherapie met combinatiebehandeling van Nivolumab en cabozantinib als eerstelijnsbehandeling voor uitgezaaide nierkanker geeft veel betere resultaten dan stndaard behandeling sunitinib wat betreft progressievrije overleving en algehele overleving
- NLR meting - veranderende verhouding van neutrofielen tot lymfocyten - blijkt een uitstekende en eenvoudige manier om de werkzaamheid van immuuntherapie met anti-PD medicijnen tijdens behandelingsfase te controleren. copy 1
- Immuuntherapie met Atezolizumab plus Avestin - bevacizumab geeft betere progressievrije ziekte en overall overleving dan sunitinib bij patienten met gevorderde uitgezaaide nierkanker
- Avelumab plus Axitinib geeft veel betere progressievrije overleving (plus 5 maanden) dan sunitinib als eerstelijns behandeling bij nog niet behandelde uitgezaaide nierkanker.
- Pembrolizumab plus Axitinib geeft betere overleving op 1 jaar (plus 11 procent) en betere progressievrije ziekte dan met sunitinib voor nog niet behandelde uitgezaaide nierkanker
- Nivolumab (Obvio) alleen en samen met ipilimumab (Yervoy) geeft uitstekende resultaten bij gevorderde niercelkanker ook in vergelijking met sunitinib
- Nivolumab een immuuntherapeutisch anti PD medicijn geeft uitstekende resultaten bij vergevorderde nierkanker versus everolimus en krijgt van FDA goedkeuring voor gebruik als medicijn
- immuuntherapie met axitinib (Inlyta®) en pembrolizumab (Keytruda®) bij gevorderde nog onbehandelde nierkanker verdubbelt progressievrije ziekte (10 vs 20 maanden)
- Immuuntherapie met rocapuldencel-T (AGS-003), een vorm van dendritische celtherapie met individuele T-cel stimulatie geeft uitstekende resultaten bij nieuwe patienten met uitgezaaide nierkanker
- Immuuntherapie bij nierkanker, een overzicht



Plaats een reactie ...
Reageer op "Clostridium butyricum (CBM 588) een probioticum toegevoegd aan nivolumab plus ipilimumab verbeterd sterk de repons en progressievrije tijd voor patienten met uitgezaaide nierkanker"