13 augustus 2021: lees ook dit artikel en zie ook in gerelateerde artikelen uiteraard: https://kanker-actueel.nl/80-procent-van-patienten-met-borstkanker-met-dics-ductaal-carcinoma-in-situ-zouden-kunnen-volstaan-met-alleen-een-operatie-maar-zonder-bestraling-post-operatief-of-zelfs-met-een-wait-and-see-beleid.html
12 november 2018: lees ook dit artikel:
28 september 2018: lees het advies van het Zorginstituut om de mammaprint uit de basisverzekering te halen.
5 juni 2018: Bron: NEJM, June 3, 2018
Het definitieve studierapport: Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer is deze week gepubliceerd in NEJM en is volledig gratis in te lezen of te downloaden.
Met als conclusie dat alleen hormoontherapie een gelijke overall overleving op 10 jaar geeft dan chemo plus hormoontherapie. De mammaprint kan voorspellen welke vrouwen wel en welke vrouwen geen chemo nodig zouden hebben.
Conclusions
Adjuvant endocrine therapy and chemoendocrine therapy had similar efficacy in women with hormone-receptor–positive, HER2-negative, axillary node–negative breast cancer who had a midrange 21-gene recurrence score, although some benefit of chemotherapy was found in some women 50 years of age or younger. (Funded by the National Cancer Institute and others; TAILORx ClinicalTrials.gov number, NCT00310180.)![]()
Zie verder alles over de mammaprint hieronder en in gerelateerde artikelen.
29 maart 2017: Bron: DOI: 10.1200/JCO.2016.70.3959 Journal of Clinical Oncology - published online before print March 13, 2017
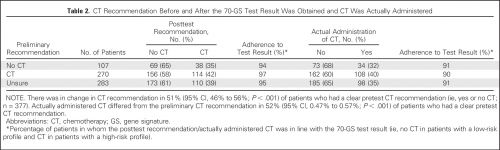
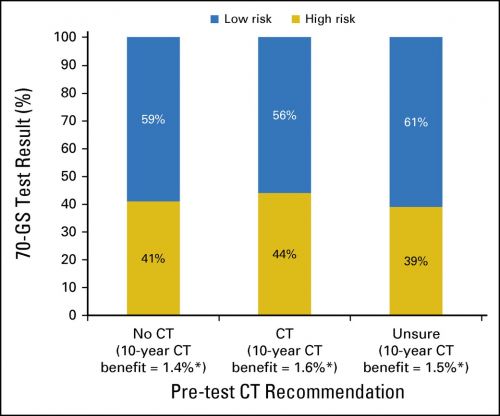
Dat ook vrouwen met hormoongevoelige borstkanker een mammaprint absoluut nodig hebben blijkt uit een nieuwe Nederlandse studie onder 660 patiënten in 33 verschillende ziekenhuizen in de periode oktober 2013 t/m december 2015. Maar liefst 51 procent kreeg na een mammaprint met de 70 genentest van de mammaprint een ander behandelplan voorgesteld dan wat de oncoloog had voorgesteld op basis van de CT-scans enz. voordat er een mammaprint werd gemaakt. En dan te bedenken dat nog maar de helft van de vrouwen met borstkanker deze mammaprint krijgen. vaak nog op eigen verzoek.
Hier de conclusie in het Engels uit het studierapport
Between October 1, 2013, and December 31, 2015, 660 patients, treated in 33 hospitals, were enrolled. Fifty-one percent of patients had pT1cN0, BRII, HER2-Neu-negative breast cancer. On the basis of conventional clinicopathological characteristics, physicians recommended CT in 270 (41%) of the 660 patients and recommended withholding CT in 107 (16%) of the 660 patients. For the remaining 43% of patients, the physicians were unsure and unable to give advice before 70-GS testing. In patients for whom CT was initially recommended or not recommended, 56% and 59%, respectively, were assigned to a low-risk profile by the 70-GS (κ, 0.02; 95% CI, -0.08 to 0.11). After disclosure of the 70-GS test result, the preliminary advice was changed in 51% of patients who received a recommendation before testing; the definitive CT recommendation of the physician was in line with the 70-GS result in 96% of patients.
Het volledige studierapport: Impact of 70-Gene Signature Use on Adjuvant Chemotherapy Decisions in Patients With Estrogen Receptor–Positive Early Breast Cancer: Results of a Prospective Cohort Study is gratis in te zien.
Hier het abstract van de studie:
use of the MammaPrint 70-gene signature (70-GS) test changed physician-intended recommendations to administer adjuvant chemotherapy in half of patients with early-stage estrogen receptor–positive breast cancer
DOI: 10.1200/JCO.2016.70.3959 Journal of Clinical Oncology - published online before print March 13, 2017
Impact of 70-Gene Signature Use on Adjuvant Chemotherapy Decisions in Patients With Estrogen Receptor–Positive Early Breast Cancer: Results of a Prospective Cohort Study
Anne Kuijer, Marieke Straver, Bianca den Dekker, Annelotte C.M. van Bommel, and Thijs van Dalen, Diakonessenhuis; Anne Kuijer, Sjoerd G. Elias, Sabine C. Linn, and Thijs van Dalen, University Medical Centre Utrecht; Sabine Siesling, Netherlands Comprehensive Cancer Organization, Utrecht; Carolien H. Smorenburg and Jelle Wesseling, Antoni van Leeuwenhoek Hospital; Carolien H. Smorenburg, Jelle Wesseling, Sabine C. Linn, and Emiel J.Th. Rutgers, Netherlands Cancer Institute, Amsterdam; and Sabine Siesling, University of Twente, Enschede, the Netherlands.
Abstract
Gene-expression profiles increasingly are used in addition to conventional prognostic factors to guide adjuvant chemotherapy (CT) decisions. The Dutch guideline suggests use of validated gene-expression profiles in patients with estrogen receptor (ER) –positive, early-stage breast cancer without overt lymph node metastases. We aimed to assess the impact of a 70-gene signature (70-GS) test on CT decisions in patients with ER-positive, early-stage breast cancer.
In a prospective, observational, multicenter study in patients younger than 70 years old who had undergone surgery for ER-positive, early-stage breast cancer, physicians were asked whether they intended to administer adjuvant CT before deployment of the 70-GS test and after the test result was available.
Between October 1, 2013, and December 31, 2015, 660 patients, treated in 33 hospitals, were enrolled. Fifty-one percent of patients had pT1cN0, BRII, HER2-Neu-negative breast cancer. On the basis of conventional clinicopathological characteristics, physicians recommended CT in 270 (41%) of the 660 patients and recommended withholding CT in 107 (16%) of the 660 patients. For the remaining 43% of patients, the physicians were unsure and unable to give advice before 70-GS testing. In patients for whom CT was initially recommended or not recommended, 56% and 59%, respectively, were assigned to a low-risk profile by the 70-GS (κ, 0.02; 95% CI, -0.08 to 0.11). After disclosure of the 70-GS test result, the preliminary advice was changed in 51% of patients who received a recommendation before testing; the definitive CT recommendation of the physician was in line with the 70-GS result in 96% of patients.
In this prospective, multicenter study in a selection of patients with ER-positive, early-stage breast cancer, 70-GS use changed the physician-intended recommendation to administer CT in half of the patients.
| 1. | Goldhirsch A, Wood WC, Coates AS, et al: Strategies for subtypes: Dealing with the diversity of breast cancer—Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736-1747, 2011 CrossRef, Medline |
| 2. | van ’t Veer LJ, Dai H, van de Vijver MJ, et al: Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530-536, 2002 CrossRef, Medline |
| 3. | van de Vijver MJ, He YD, van’t Veer LJ, et al: A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999-2009, 2002 CrossRef, Medline |
| 4. | Buyse M, Loi S, van’t Veer L, et al: Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98:1183-1192, 2006 CrossRef, Medline |
| 5. | Bueno-de-Mesquita JM, Linn SC, Keijzer R, et al: Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat 117:483-495, 2009 CrossRef, Medline |
| 6. | Mook S, Schmidt MK, Viale G, et al: The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1-3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat 116:295-302, 2009 CrossRef, Medline |
| 7. | Knauer M, Mook S, Rutgers EJ, et al: The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 120:655-661, 2010 CrossRef, Medline |
| 8. | Mook S, Schmidt MK, Weigelt B, et al: The 70-gene prognosis signature predicts early metastasis in breast cancer patients between 55 and 70 years of age. Ann Oncol 21:717-722, 2010 CrossRef, Medline |
| 9. | Mook S, Knauer M, Bueno-de-Mesquita JM, et al: Metastatic potential of T1 breast cancer can be predicted by the 70-gene MammaPrint signature. Ann Surg Oncol 17:1406-1413, 2010 CrossRef, Medline |
| 10. | Bueno-de-Mesquita JM, van Harten WH, Retel VP, et al: Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: A prospective community-based feasibility study (RASTER). Lancet Oncol 8:1079-1087, 2007 CrossRef, Medline |
| 11. | Drukker CA, Bueno-de-Mesquita JM, Retèl VP, et al: A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer 133:929-936, 2013 CrossRef, Medline |
| 12. | Cardoso F, van’t Veer LJ, Bogaerts J, et al: 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717-729, 2016 CrossRef, Medline |
| 13. | Kwaliteitsinstituut voor de gezondheidszorg CBO VvIK. Risicoprofilering. Richtlijn mammacarcinoom 81-83, 2012 |
| 14. | Senkus E, Kyriakides S, Penault-Llorca F, et al: Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi7-vi23, 2013 (suppl 6) CrossRef, Medline |
| 15. | Kuijer A, van Bommel AC, Drukker CA, et al: Using a gene expression signature when controversy exists regarding the indication for adjuvant systemic treatment reduces the proportion of patients receiving adjuvant chemotherapy: A nationwide study. Genet Med 18:720-726, 2016 CrossRef, Medline |
| 16. | Kuijer A, Drukker CA, Smorenburg C, et al. Change over time in the impact of gene-expression profiles on the administration of adjuvant CT. Int J Cancer 139:769-775, 2016 |
| 17. | Wishart GC, Bajdik CD, Dicks E, et al: PREDICT Plus: Development and validation of a prognostic model for early breast cancer that includes HER2. Br J Cancer 107:800-807, 2012 CrossRef, Medline |
| 18. | Drukker CA, Nijenhuis MV, Bueno-de-Mesquita JM, et al: Optimized outcome prediction in breast cancer by combining the 70-gene signature with clinical risk prediction algorithms. Breast Cancer Res Treat 145:697-705, 2014 CrossRef, Medline |
| 19. | Marshall DA, Deal K, Bombard Y, et al: How do women trade-off benefits and risks in chemotherapy treatment decisions based on gene expression profiling for early-stage breast cancer? A discrete choice experiment. BMJ Open 6:e010981, 2016 CrossRef, Medline |
| 20. | DeFrank JT, Carey LA, Brewer NT: Understanding how breast cancer patients use risk information from genomic tests. J Behav Med 36:567-573, 2013 CrossRef, Medline |
| 21. | Exner R, Bago-Horvath Z, Bartsch R, et al: The multigene signature MammaPrint impacts on multidisciplinary team decisions in ER+, HER2− early breast cancer. Br J Cancer 111:837-842, 2014 CrossRef, Medline |
| 22. | Cusumano PG, Generali D, Ciruelos E, et al: European inter-institutional impact study of MammaPrint. Breast 23:423-428, 2014 CrossRef, Medline |
| 23. | Torrisi R, Garcia-Etienne CA, Losurdo A, et al: Potential impact of the 70-gene signature in the choice of adjuvant systemic treatment for ER positive, HER2 negative tumors: A single-institution experience. Breast 22:419-424, 2013 CrossRef, Medline |
| 24. | Carlson JJ, Roth JA: The impact of the Oncotype Dx breast cancer assay in clinical practice: A systematic review and meta-analysis. Breast Cancer Res Treat 141:13-22, 2013 CrossRef, Medline |
| 25. | Martín M, González-Rivera M, Morales S, et al: Prospective study of the impact of the Prosigna assay on adjuvant clinical decision-making in unselected patients with estrogen receptor positive, human epidermal growth factor receptor negative, node negative early-stage breast cancer. Curr Med Res Opin 31:1129-1137, 2015 CrossRef, Medline |
| 26. | Wuerstlein R, Sotlar K, Gluz O, et al: The West German Study Group breast cancer intrinsic subtype study: A prospective multicenter decision impact study utilizing the Prosigna assay for adjuvant treatment decision-making in estrogen-receptor-positive, HER2-negative early-stage breast cancer. Curr Med Res Opin 32:1217-1224, 2016 CrossRef, Medline |
| 27. | Levine MN, Julian JA, Bedard PL, et al: Prospective evaluation of the 21-gene recurrence score assay for breast cancer decision-making in Ontario. J Clin Oncol 34:1065-1071, 2016 Link |
Gerelateerde artikelen
- Mammaprint voor borstkankerpatienten gaat vergoed worden vanuit basisverzekering laat het Zorginstituut weten. De mindact studie toont aan dat minder vrouwen met borstkanker chemotherapie nodig hebben
- Mammaprint verandert voorgestelde behandeling van hormoongevoelige borstkanker bij 51 procent van de vrouwen in vergelijking met behandelplan zonder mammaprint.
- Mammaprint bewijst grote waarde voor borstkankerpatienten. 50 procent van borstkankerpatienten hoeft geen chemo. RASTER studie nu officieel gepubliceerd
- Uitstekende resultaten met mammaprint blijkt uit Nederlands onderzoek
- Mammaprint (R) wordt meer en meer gebruikt bij behandelplan voor borstkankerpatienten. Achmea gaat dit nu ook standaard vergoeden.
- Mammaprint: Onderzoek met micro-arrays (genendiagnostiek bij borstkanker) geeft uitstekende prognose op overleving en succesvol toe te passen behandelingen in vergelijking met klassiekere prognosetesten
- Mammaprint voor bepaling van de kansen van een recidief van borstkanker te verkrijgen bij commercieel instituut in Nederland
- Mammaprint - genentest bewijst waarde voor borstkanker en kan ook uitgevoerd worden op bewaard tumorweefsel.



Plaats een reactie ...
Reageer op "Mammaprint verandert voorgestelde behandeling van hormoongevoelige borstkanker bij 51 procent van de vrouwen in vergelijking met behandelplan zonder mammaprint."