Helpt u ons aan 500 donateurs?
17 september 2018: lees ook het ervaringsverhaal van meneer Cor C.
Lees ook:
en wellicht ook dit artikel:
https://kanker-actueel.nl/NL/probiotica-melkzuurbacterien-vooraf-en-na-operaties-bij-darmkanker-voorkomen-ernstige-infecties-bevorderen-sneller-herstel-en-zorgen-voor-kortere-ziekenhuisopname.html
Update 5 januari 2020: Vergelijkingsstudie van kortstondige radiotherapie gevolgd door chemotherapie vóór TME = totale mesorectale excisie versus preoperatieve chemoradiotherapie, TME en optionele adjuvante chemotherapie bij lokaal gevorderde rectumkanker toont aan dat een totale preoperatieve aanpak veel beter post operatieve uitzaaiingen op afstand voorkomt. Hoewel er statistisch geen verschil zat in overall overleving blijkt het verschil in effectiviteit van de behandelingen op uitzaaiingen op afstand op 3-jaars meting 23,7% versus 30,4%.
Met ongeveer 450 patiënten per groep zagen de auteurs een verbeterde pathologische complete respons en minder ziektegerelateerde mislukte behandelingen in hun experimentele (TNT) groep, voornamelijk als gevolg van minder metastasen op afstand in de experimentele groep (86/128 [67%] versus in de standaardgroep (123/152 [81%].
De onderzoekers stellen dan ook dat deze bevindingen de experimentele benadering van totale neoadjuvante therapie als standaardzorg voor deze patiëntenpopulatie algemeen standaard zou moeten zijn en worden.
Het volledige studierapport is gepubliceerd in The Lancet en moet voor betaald worden: Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial (abstract onderaan artikel)
27 april 2018: Bron: EJSO - European Journal of Surgical Oncology
Patiënten met lokaal gevorderde rectumkanker (LARC) (stadium III, dus hoog risico op een recidief) die om wat voor reden dan ook (meestal slechte lichamelijke conditie) ongeschikt zijn voor de standaard behandeling chemoradiatie (CRT), worden vaak een korter durende periode radiotherapie aangeboden gevolgd door een zogeheten SCRT-delay = kortdurende bestraling met een vertraagde operatie, dus niet dirct na de bestraling. Deze SCRT-delay behandeling houdt een lagere stralingsdosis in, geen chemotherapie en een kortere behandelingsperiode. Een Nederlandse studie heeft op basis van de landelijke registratiedatabase geanalyseerd wat in de periode van 2008 tot 2014 de verschillen waren in bereikte pathologische complete remissies (pCR = klinisch kankervrij) tussen een volledige CRT en de SCRT-delay behandeling bij patiënten met lokaal gevorderde rectumkanker (endeldarmkanker) LARC.
En die resultaten blijken voor de SCRT-delay beduidend slechter dan voor de CRT.
Van de 386 patiënten die een SCRT-delay behandeling hadden gehad bereikte slechts 6,4 procent een pathologische complete remissie tegenover 16,2 van de 3659 patiënten die een volledige CRT hadden gehad. (6.4% vs. 16.2%, p < 0.001)
Na correctie voor tumorstadium, aangetaste lymfklieren en periode voor operatie plaatsvond, hadden patiënten die een SCRT-delay behandeling hadden gehad statistisch significant minder kans om een pCR te bereiken (aangepaste oddsratio 0,3, 95% BI 0,2-0,5).
Ik moet zeggen dat dit toch wel hele lage percentages zijn voor zulke belastende behandelingen.
En is ook wel in contrast met deze Poolse studie al uit 2006 Doen ze het dan in Nederland zo slecht? Ik begrijp niet waarom de verschillen tussen bovengenoemde Poolse studie en de Nederlandse studie de resultaten zo ver uit elkaar liggen. 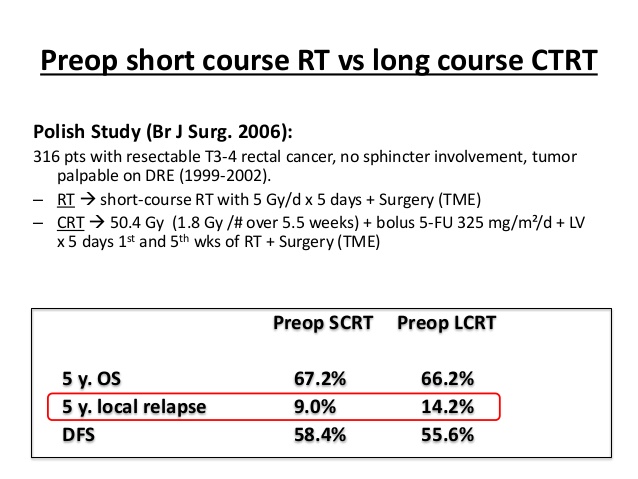
Uit het studierapport: Comparison of pathological complete response rates after neoadjuvant short-course radiotherapy or chemoradiation followed by delayed surgery in locally advanced rectal cancer dat gratis is in te zien hier enkele grafieken. Het abstract staat daaronder met referentielijst.
Table 1 geeft de karakteristieken van de patienten weer. Daaronder table 2 die de resultaten op pathologische complete remissies weergeeft.
| SCRT-delay N = 391 (%) | CRT N = 3659 (%) | |
|---|---|---|
| Mean age in years ± SD | 76 ± 9 | 63 ± 10 |
| Sex | ||
| Male | 198 (50.6) | 2338 (63.9) |
| Female | 193 (49.4) | 1321 (36.1) |
| Tumor distance | ||
| ≤5 cm | 176 (45.0) | 1680 (45.9) |
| 6–10 cm | 146 (37.3) | 1324 (36.2) |
| ≥11 cm | 43 (11.0) | 437 (11.9) |
| Missing | 26 (6.6) | 218 (6.0) |
| cT-stage | ||
| 2 | 13 (3.3) | 143 (3.9) |
| 3 | 298 (76.2) | 2769 (75.7) |
| 4 | 80 (20.5) | 747 (20.4) |
| cN-stage | ||
| 0 | 37 (9.5) | 192 (5.2) |
| 1 | 216 (55.2) | 1569 (42.9) |
| 2 | 133 (34.0) | 1840 (50.3) |
| Missing | 5 (1.3) | 58 (1.6) |
| Median time interval (IQR) | 9.1 (6.9–12.0) | 9.4 (8.0–11.3) |
| Time interval categorical | ||
| ≤7 weeks | 106 (27.1) | 533 (14.6) |
| 8–9 weeks | 87 (22.3) | 1090 (29.8) |
| 10–11 weeks | 72 (18.4) | 1034 (28.3) |
| ≥12 weeks | 126 (32.2) | 1002 (27.4) |
| Surgical procedure | ||
| TEM/TAE | 5 (1.3) | 18 (0.5) |
| LAR | 146 (37.3) | 1768 (48.3) |
| APR | 169 (43.2) | 1498 (40.9) |
| Intersphincteric resection | 10 (2.6) | 34 (0.9) |
| Hartmann resection | 56 (14.3) | 270 (7.4) |
| Sigmoid resection | 1 (0.3) | 3 (0.1) |
| Extended TME | 4 (1.0) | 68 (1.9) |
| Vital status | ||
| Alive | 268 (68.5) | 3021 (82.6) |
| Dead | 123 (31.5) | 638 (17.4) |
| Median years follow-up (IQR) | 2.4 (1.5–3.5) | 3.2 (2.0–4.7) |
APR: abdominoperineal resection; CRT: chemoradiation; LAR: low anterior resection; SCRT: short-course radiotherapy.
TAE: transanal excision; TEM: Transanal endoscopic microsurgery; TME: total mesorectal excision.
| SCRT-delay N = 391 (%) | CRT N = 3659 (%) | p-value | |
|---|---|---|---|
| pCR (ypT0-N0) | 25 (6.4) | 592 (16.2) | <0.001 |
| Near-pCR (ypT0–1 N0) | 43 (11.0) | 755 (20.6) | <0.001 |
| Tumor downstaging (ypT < cT) | 182 (46.8) | 2079 (58.1) | <0.001 |
| Nodal downstaging (ypN < cN) | 225 (58.1) | 2618 (72.4) | <0.001 |
| ypT-stage | <0.001 | ||
| 0 | 31 (7.9) | 673 (18.4) | |
| 1 | 21 (5.4) | 210 (5.7) | |
| 2 | 99 (25.3) | 934 (25.5) | |
| 3 | 206 (52.7) | 1581 (43.2) | |
| 4 | 32 (8.2) | 183 (5.0) | |
| Missing | 2 (0.5) | 78 (2.1) | |
| ypN-stage | <0.001 | ||
| 0 | 215 (55.0) | 2413 (65.9) | |
| 1 | 109 (27.9) | 805 (22.0) | |
| 2 | 63 (16.1) | 400 (10.9) | |
| Missing | 4 (1.0) | 41 (1.1) | |
| Median no. of examined lymph nodes (IQR) | 14 (11–19) | 12 (9–16) | <0.001a |
| Median lymph node ratio (IQR)b | 0 (0–0.09) | 0 (0–0.14) | <0.001a |
CRT: chemoradiation; pCR: pathological complete response; SCRT: short-course radiotherapy.
Compared to patients treated with neoadjuvant CRT, those receiving SCRT and delayed surgery are less likely to develop pCR. Novel neoadjuvant treatment strategies for patients not fit enough for CRT are needed to increase their eligibility for organ-sparing treatments.
Source: EJSO - European Journal of Surgical Oncology
INTRODUCTION
Patients with locally advanced rectal cancer (LARC) who are unfit for chemoradiation (CRT), are often offered short-course radiotherapy followed by delayed surgery (SCRT-delay). This entails a lower radiation dose, no chemotherapy and a shorter treatment period. This may lower their chances for complete tumor response and, as such, organ-sparing approaches. The purpose of this study was to compare the pathological complete response (pCR) rates between neoadjuvant CRT and SCRT-delay in patients with LARC in a nationwide database from the Netherlands.
METHODS
In the population based Netherlands Cancer Registry, clinical stage III rectal cancer patients, diagnosed between 2008 and 2014, who underwent CRT or SCTR-delay were selected. pCR (ypT0N0), near pCR (ypT0-1N0), and tumor and nodal downstaging were compared between the treatment groups using multivariable logistic regression analysis.
RESULTS
386 patients underwent SCRT-delay and 3659 patients underwent CRT. The pCR-rate in the SCRT-delay group was significantly lower compared to the CRT-group (6.4% vs. 16.2%, p < 0.001). After adjustment for clinical tumor stage, clinical nodal stage and time interval to surgery, SCRT-delay patients were significantly less likely to reach pCR (adjusted odds ratio 0.3, 95%CI 0.2-0.5). Also, near-pCR (ypT0-1N0) as well as tumor and nodal downstaging was observed less often in the SCRT-delay group.
CONCLUSION
Compared to patients treated with neoadjuvant CRT, those receiving SCRT and delayed surgery are less likely to develop pCR. Novel neoadjuvant treatment strategies for patients not fit enough for CRT are needed to increase their eligibility for organ-sparing treatments.
Discussion
Pathological compete response rates are lower in patients treated with neoadjuvant short-course radiotherapy with delayed surgery than in patients treated with neoadjuvant chemoradiation for locally advanced rectal cancer. This is far from unexpected, since the administered biological effective radiation dose is lower in SCRT. SCRT followed by surgery within 10 days does not induce downstaging, let alone pCR [19]. Moreover, since chemotherapy is eliminated, the lack of a radiosensitizing agent may reduce radiotherapy efficacy [20]. A prolonged interval to surgery increases the pCR rate [, ]. In previous studies the pCR rates following SCRT-delay range from 4.4% to 25%, with intervals to surgery from 4 up to 19 weeks [, , , , , , , , ]. A systematic review found an average increase in pCR rate of 10% in the delayed-surgery group compared to immediate surgery (3–12% and 0–0.4%, respectively) [21]. The Stockholm III trial showed that prolonging the interval to surgery from 1 week to 4–8 weeks increases pCR rates from 2.1% to 11.8% [16]. No differences were found in local recurrence, recurrence-free survival and 5-year overall survival [30]. A comparison between SCRT-delay and CRT for cT1-4 N0-3 tumors showed clinical complete response (cCR) rates of 20% and 34% after a median interval to response evaluation of 10.3 and 8.9 weeks, respectively. cCR was only achieved in cT2N0 and cT3N0 tumors [31]. Similar effects on cT3 tumors have been published [, ]. In our study, pCR rate was 6.5% after SCRT-delay, with a mean interval to surgery of 9.1 weeks. This relatively low pCR rate may be attributed to higher tumors stages (Stage III or cT4N0) in our study population.
In contrast to literature, we did not observe a higher pCR rates when interval to surgery was increased within the CRT group. This corresponds to the results of the phase III randomized GRECCAR-6 trial, where no benefit of a prolonged interval beyond a minimal interval of 11 weeks was found [32].
We observed a lower proportion of near-pCR (ypT0-1) in the SCRT-delay group than in the CRT group. Near-pCR is relevant when local excision as an alternative treatment option is considered, for example for frail patients, to reduce acute and long-term morbidity of surgery, such as anastomotic leakage, bleeding, urinary, and fecal incontinence [, , ].
A higher pathological nodal stage, a higher mean number of examined lymph nodes and a higher mean metastatic/examined lymph nodes ratio (LNR) were observed in the SCRT-delay group compared to the CRT-group. An increase in lymph node yield after neoadjuvant therapy for locally advanced rectal cancer is associated with improved cancer-specific and 5-year survival and lower local recurrence rates [, ]. However, in tumors with (near) pCR a lower number of lymph nodes is found [38]. A high LNR is prognostic for decreased overall and disease-free survival, even in patients with fewer than 12 lymph nodes examined [39]. These findings suggest that prognosis after SCRT-delay for LARC might be worse than after CRT, but the clinical relevance of LNR is still under debate.
To the best of our knowledge, this is the first large, nationwide study that describes the difference in pathological response rates between chemoradiation and short-course radiotherapy in LARC. A limitation of our study is the missing information about involvement of the mesorectal fascia (MRF). According to Dutch Guidelines, SCRT might have been justified in cT3N1 patients with a wide margin (>1 mm) to the MRF. An increased interval to surgery could have increased pCR rates in this group. However, since cT3N1 MRF- patients require immediate surgery after SCRT and we selected an interval to surgery of more than 4 weeks, we assumed most of the cT3N1 in our dataset had an involved MRF and thus needed CRT.
The group of patients that receive SCRT-delay instead of CRT is not clearly defined. Studies have shown that treatment deviation is more common in elderly patients [, , , , , , ], with age and comorbidities being predictive factors for altered treatment [, , ]. Since rectal cancer incidence increases with age and the general population is getting older, clinicians increasingly consider alternative, less aggressive treatment options for the frail LARC patient. More importantly, since per- and postoperative morbidity and mortality rates are increased in the older patient due to complications [, , , ] and postoperative complications have a larger negative impact on physical- and role functioning in older patients [49], organ preservation would be favorable in this particular population. However, if SCRT-delay is mostly offered to elderly patients, and pCR rates are lower after SCRT-delay, elderly treated with SCRT-delay have a lower probability to become eligible for organ-sparing approaches. Novel neoadjuvant treatment strategies for frail patients are needed in order to increase their eligibility for organ-sparing treatments, while focusing on a balance between morbidity on the one hand and optimal function and cure rates on the other. Current studies investigate a combination of short-course radiotherapy and chemotherapy, resulting in increased pCR rates [, , , , ]. However, the risk of toxicity associated with this strategy is likely to be unacceptable in the frail rectal cancer patient. Future studies should look into new treatment modalities where therapy doses can be increased to a level where response rates are higher and toxicity is still tolerable.
Conclusion
Compared to patients treated with neoadjuvant CRT, those receiving SCRT and delayed surgery are less likely to develop pCR. Novel neoadjuvant treatment strategies for patients not fit enough for CRT are needed to increase their eligibility for organ-sparing treatments.
Disclosures
None.
Funding
None.
Conflict of interest
None.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice.
The observed decreased probability of disease-related treatment failure in the experimental group is probably indicative of the increased efficacy of preoperative chemotherapy as opposed to adjuvant chemotherapy in this setting. Therefore, the experimental treatment can be considered as a new standard of care in high-risk locally advanced rectal cancer.
Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial
- et al.
Summary
Background
Methods
Findings
Interpretation
Funding
Summary
Background
Methods
Findings
Interpretation
Funding
Gerelateerde artikelen
- Verkorte radiotherapie vooraf aan operatie (TME) bij lokaal uitgezaaide rectumkanker geeft beduidend minder complete remissies in vergelijking met volledige chemoradiotherapie vooraf aan operatie
- Bestraling vooraf aan operatie van rectumkanker geeft significant minder kans op recidief dan alleen operatie - TME, aldus Nederlandse langjarige gerandomiseerde studie
- TME-Totale Mesorectale Exicisie (met of zonder bestraling vooraf): Nieuwe operatietechniek bij endeldarmkanker voorkomt stoma en recidief bij 97,6 procent van de operatief te helpen patiënten als er vooraf bestraald wordt.
- TME-Totale Mesorectale Exicisie met bestraling vooraf leidt tot significant grotere niet aan kanker gerelateerde sterfte binnen 6 maanden na TME bij oudere patienten (>75 jaar) met rectumkanker.
- Het effect van pre-operatieve bestraling bij TME van endeldarmkanker (TME) op kwaliteit van leven en sexueel functioneren geeft feitelijk gezien behoorlijk ernstige bijwerkingen maar wordt niet zo ervaren
- Korte periode van preoperatieve bestraling voorkomt hoog significant een recidief (61 procent) in vergelijking met postoperatieve bestraling + chemo bij geselecteerde patienten bij operabele endeldarmkanker. Blijkt u
- TME - Rectumkanker: een overzicht van studies en informatie over TME - Totale Mesorectale Exicisie



Plaats een reactie ...
Reageer op "Verkorte radiotherapie vooraf aan operatie (TME) bij lokaal uitgezaaide rectumkanker geeft beduidend minder complete remissies in vergelijking met volledige chemoradiotherapie vooraf aan operatie"