30 oktober 2017: Bron: European Urology: August 2015 Volume 68, Issue 2, Pages 267–279
Bij nagenoeg alle vormen van kanker lijkt immuuntherapie de sleutel tot langdurige overleving. Bijna elke week wordt wel ergens een studie gepubliceerd, meestal met anti-PD medicijnen / check pointremmers maar ook andere vormen van immuuntherapie met targeted medicine,, medicijnen die zich richten op 1 of enkele mutaties of ook vormen van dendritische celtherapie met aanvullende immuunstimulerende middelen waaronder gemoduleerde virussen. Ik probeer dit bij te houden maar is onmogelijk door de veelheid alle belangrijke studies ook te publiceren met commentaar.
Omdat ik naast de website bijhouden ook nog enkele patiënten begeleid kwam ik op deze studie: A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G 
die opgemaakt eind 2014 en gepubliceerd in 2015 een overzicht geeft van immuuntherape bij urologische vormen van kanker, zoals blaaskanker,prostaatkanker en nierkanker.
De studie is dus al weer 2 jaar oud en er zijn al weer veel aanvullingen te maken, maar voor de meeste studies moet betaald worden voor een volledig studierapport. En deze studie geeft wel een goed overzicht van hoe immuuntherapie precies werkt, welke bepaalde mutaties een rol daarbij spelen en welke ontwikkelingen er plaatsvinden bij urologische vormen van kanker. En is uitgeplitst in de verschillende vormen van immuuntherapie en beschrijft het tot nu toe gedaan onderzoek bij respectievelijk blaaskanker, prostaatkanker en nierkanker.
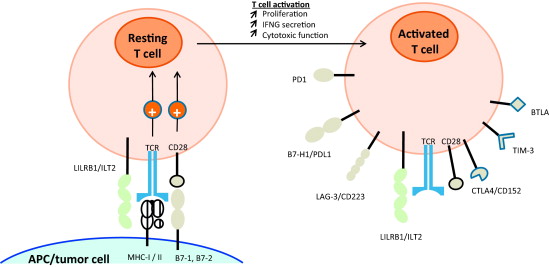
Hier enkele citatn uit de conclusie van deze reviewstudie:
An improved understanding of the molecular mechanisms that govern interactions between a tumor and the host immune response has led to major advances in targeted immunotherapy and cancer treatments. Our systematic review demonstrates that immune checkpoint inhibitors offer interesting and long-lasting response rates in heavily pretreated patients with advanced urologic cancers.
In prostate cancer, a growing body of data supports the oncologic role of anti–CTLA-4 antibodies, alone or in combination with other immune therapies. ........
In renal cancer, the most encouraging findings have been observed for PD-1/PD-L1 inhibitors given their safety and antitumor activity. .......
The field of immunotherapy in urologic cancer treatment is evolving. Oncologic efficacy, including prolongation of overall survival, has already been observed for immune checkpoint inhibitors in various malignancies, mainly in breast cancer and melanomas. Several ongoing trials are studying immune therapy combinations and immune monotherapy combined with conventional anticancer drugs (Table 4). An anti–CTLA-4 antibody and vaccine combination has already been tested with interesting outcomes. Preclinical studies also support the synergistic role of CTLA-4 and PD-1 blockade [, ].
Table 4 laat lopende studies zien, maar voor recente studies kunt u het beste kijken in clinicaltrials. :
| Clinical trial | Drug | Phase | Cancer | Estimated enrollment | Population | Primary endpoint | Arms | Estimated completion date |
|---|---|---|---|---|---|---|---|---|
| CTLA-4 | ||||||||
| NCT01057810 | Ipilimumab | 3 | Prostate | 60 | CT-naïve mCRPC | OS | Versus placebo | February 2016 |
| NCT01530984 | Ipilimumab | 2 | Prostate | 54 | CT-naïve mCRPC | PSA decline | Versus ipilimumab + GM-CSF | December 2018 |
| NCT01688492 | Ipilimumab | 1/2 | Prostate | 25 | CT-naïve mCRPC | PSA decline | With abiraterone | September 2015 |
| NCT01498978 | Ipilimumab | 2 | Prostate | 30 | mCRPC | PSA decline | April 2018 | |
| NCT01194271 | Ipilimumab | 2a | Prostate | 20 | Localized | Immune response | Neoadjuvant | September 2015 |
| NCT01804465 | Ipilimumab | 2 | Prostate | 66 | CT-naïve mCRPC | Immune response | With sipuleucel-T | December 2016 |
| NCT02231749 | Ipilimumab | 3 | RCC | 1070 | mRCC | OS, PFS | With nivolumab versus sunitinib | October 2019 |
| NCT01524991 | Ipilimumab | 2 | Bladder | 36 | M | OS | With GC | June 2016 |
| PD-1 | ||||||||
| NCT01354431 | Nivolumab | 2 | RCC | 150 | mRCC | RECIST | April 2015 | |
| NCT01668784 | Nivolumab | 3 | RCC | 822 | mRCC | RECIST | Versus everolimus | September 2016 |
| NCT01441765 | Pidilizumab | 2 | RCC | 44 | mRCC | RECIST | ± dendritic cell vaccine | November 2015 |
| NCT01420965 | Pidilizumab | 2 | Prostate | 57 | mCRPC | Immune response | With sipuleucel ± cyclophosphamide | December 2018 |
| NCT02210117 | Nivolumab | 2 | RCC | 45 | mCRPC | Safety | ± sunitinib versus ± ipilimumab | January 2019 |
| NCT01928394 | Nivolumab | 1/2 | Bladder | 410 | M | Response rate | ± ipilimumab | March 2017 |
| PD-L1 | ||||||||
| NCT01984242 | MPDL3280A | 2 | RCC | 150 | mRCC | RECIST | ± sunitinib versus bevacizumab | January 2016 |
| NCT02108652 | MPDL3280A | 2 | Bladder | 330 | M | Response rate | March 2016 | |
| B7-H3 | ||||||||
| NCT01391143 | MGA271 | 1 | Prostate Bladder RCC |
151 | M | Safety | February 2016 | |
deze studie: A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G  die opgemaakt eind 2014 en gepubliceerd in 2015.
die opgemaakt eind 2014 en gepubliceerd in 2015.
Hier het abstract plus referentielijst:
Data from studies support the activity and safety of immune checkpoint inhibitors in urologic cancers, alone or in combination with conventional cancer therapies. Encouraging data in other oncologic fields could translate into interesting responses in urological cancers.
A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G ☆
☆
DOI: http://dx.doi.org/10.1016/j.eururo.2015.02.032
Article Outline
Abstract
Context
Overexpression of immune checkpoint molecules affects tumor-specific T-cell immunity in the cancer microenvironment, and can reshape tumor progression and metastasis. Antibodies targeting checkpoints could restore antitumor immunity by blocking the inhibitory receptor-ligand interaction.
Objective
To analyze data and current trends in immune checkpoint targeting therapy for urologic cancers.
Evidence acquisition
Systematic literature search for clinical trials in the PubMed and Cochrane databases up to August 2014 according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. Endpoints included oncologic results, tumor response rates, safety, and tolerability.
Evidence synthesis
Anti-CTLA-4 monotherapy has demonstrated biochemical responses in prostate cancer. One phase 3 trial assessing ipilimumab efficacy in castration-resistant disease was negative overall. Nevertheless, ipilimumab may significantly improve overall survival compared with placebo in subgroups of patients with favorable prognostic features. In renal cancer, phase 1 trials showed interesting stabilization or long-lasting objective response rates approaching 50% using anti-PD-1/PD-L1 drugs in heavily pretreated metastatic patients. In bladder cancer, one phase 2 trial indicated a good safety profile for ipilimumab as a neoadjuvant drug before radical cystectomy. Overall, immune-related effects such as colitis and dermatitis were common and well tolerated.
Conclusions
Our systematic review shows that antibodies blocking immune checkpoints offer interesting and long-lasting response rates in heavily pretreated patients with advanced urologic cancers. More promising results are currently provided by anti-CTLA-4 antibodies in prostate cancer and by PD-1/PD-L1 inhibitors in renal cancer. These should encourage new clinical trials of immune therapy combinations and immunotherapy monotherapy combined with conventional anticancer drugs. In bladder cancer, the use of targeted immunotherapy still remains underevaluated; however, preliminary results reported at recent conferences seem encouraging.
Patient summary
Data from studies support the activity and safety of immune checkpoint inhibitors in urologic cancers, alone or in combination with conventional cancer therapies. Encouraging data in other oncologic fields could translate into interesting responses in urological cancers.
References
- Collins, A.V., Brodie, D.W., Gilbert, R.J.C. et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002; 17: 201–210
- Rudd, C.E., Taylor, A., and Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009; 229: 12–26
- Teft, W.A., Kirchhof, M.G., and Madrenas, J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006; 24: 65–97
- Lenschow, D.J., Walunas, T.L., and Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996; 14: 233–258
- Wing, K., Onishi, Y., Prieto-Martin, P. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008; 322: 271–275
- Hurwitz, A.A., Foster, B.A., Kwon, E.D. et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000; 60: 2444–2448
- Demaria, S., Kawashima, N., Yang, A.M. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005; 11: 728–734
- Wada, S., Jackson, C.M., Yoshimura, K. et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013; 11: 89
- Slovin, S.F., Higano, C.S., Hamid, O. et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013; 24: 1813–1821
- Small, E.J., Tchekmedyian, N.S., Rini, B.I., Fong, L., Lowy, I., and Allison, J.P. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007; 13: 1810–1815
- Fong, L., Kwek, S.S., O’Brien, S. et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009; 69: 609–615
- Madan, R.A., Mohebtash, M., Arlen, P.M. et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012; 13: 501–508
- Jochems, C., Tucker, J.A., Tsang, K.Y. et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunother. 2014; 63: 407–418
- van den Eertwegh, A.J.M., Versluis, J., van den Berg, H.P. et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012; 13: 509–517
- Santegoets, S.A.M., Stam, A.M., Lougheed, S. et al. T cell profiling reveals high CD4+CTLA-4+ T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013; 62: 245–256
- Kwon, E.D., Drake, C.G., Scher, H.I. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014; 15: 700–712
- McNeel, D.G., Smith, H.A., Eickhoff, J.C. et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012; 61: 1137–1147
- Yang, J.C., Hughes, M., Kammula, U. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007; 30: 825–830
- Blansfield, J.A., Beck, K.E., Tran, K. et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005; 28: 593–598
- Rini, B.I., Stein, M., Shannon, P. et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011; 117: 758–767
- Wang, L., Su, G., Zhao, X. et al. Association between the cytotoxic T-lymphocyte antigen 4 +49A/G polymorphism and bladder cancer risk. Tumour Biol. 2014; 35: 1139–1142
- Carthon, B.C., Wolchok, J.D., Yuan, J. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010; 16: 2861–2871
- Freeman, G.J., Long, A.J., Iwai, Y. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192: 1027–1034
- Tseng, S.-Y., Otsuji, M., Gorski, K. et al. B7-Dc, a New dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001; 193: 839–846
- Ishida, Y., Agata, Y., Shibahara, K., and Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992; 11: 3887–3895
- Keir, M.E., Butte, M.J., Freeman, G.J., and Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26: 677–704
- Dong, H., Strome, S.E., Salomao, D.R. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8: 793–800
- Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S., and Honjo, T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013; 14: 1212–1218
- Topalian, S.L., Hodi, F.S., Brahmer, J.R. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366: 2443–2454
- Sfanos, K.S., Bruno, T.C., Meeker, A.K., De Marzo, A.M., Isaacs, W.B., and Drake, C.G. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate. 2009; 69: 1694–1703
- Roth, I., Corry, D.B., Locksley, R.M., Abrams, J.S., Litton, M.J., and Fisher, S.J. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996; 184: 539–548
- Tang, P.A. and Heng, D.Y. Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer. Curr Oncol Rep. 2013; 15: 98–104
- Thompson, R.H., Dong, H., and Kwon, E.D. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. (709s–15s)Clin Cancer Res. 2007; 13
- Krambeck, A.E., Dong, H., Thompson, R.H. et al. Survivin and B7-H1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007; 13: 1749–1756
- Brahmer, J.R., Drake, C.G., Wollner, I. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010; 28: 3167–3175
- Brahmer, J.R., Tykodi, S.S., Chow, L.Q. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366: 2455–2465
- Cho, D.C., Sosman, J.A., Sznol, M. et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC). ASCO Meeting Abstr. 2013; 31: 4505
- Motzer, R.J., Rini, B.I., McDermott, D.F. et al. Randomized, dose-ranging phase II trial of nivolumab for metastatic renal cell carcinoma. Ann Oncol. 2014; 25: iv281
- Amin, A., Plimack, E.R., Infante, J.R. et al. Nivolumab in combination with sunitinib or pazopanib in patients with metastatic renal cell carcinoma. (iv362–3)Ann Oncol. 2014; 25
- Choueiri, T.K., Figueroa, D.J., Fay, A.P. et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015; 21: 1071–1077
- Herbst, R.S., Soria, J.-C., Kowanetz, M. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014; 515: 563–567
- Nakanishi, J., Wada, Y., Matsumoto, K., Azuma, M., Kikuchi, K., and Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007; 56: 1173–1182
- Inman, B.A., Sebo, T.J., Frigola, X. et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007; 109: 1499–1505
- Xylinas, E., Robinson, B.D., Kluth, L.A. et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol. 2014; 40: 121–127
- Boorjian, S.A., Sheinin, Y., Crispen, P.L. et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008; 14: 4800–4808
- Plimack, E.R., Gupta, S., Bellmunt, J. et al. A phase Ib study of pembrolizumab in patients with advanced urothelial tract cancer. Ann Oncol. 2014; 25: mdu438.24
- Bellmunt, J., Petrylak, D.P., Powles, T. et al. Inhibition of PD-L1 by MPDL3208a leads to clinical activity in patients with metastatic urothelial bladder cancer. Ann Oncol. 2014; 25: iv280
- Powles, T., Eder, J.P., Fine, G.D. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014; 515: 558–562
- Carosella, E.D., Favier, B., Rouas-Freiss, N., Moreau, P., and Lemaoult, J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008; 111: 4862–4870
- Carosella, E.D., Gregori, S., and LeMaoult, J. The tolerogenic interplay(s) among HLA-G, myeloid APCs, and regulatory cells. Blood. 2011; 118: 6499–6505
- Carosella, E.D., Moreau, P., Lemaoult, J., and Rouas-Freiss, N. HLA-G: from biology to clinical benefits. Trends Immunol. 2008; 29: 125–132
- Rouas-Freiss, N., Moreau, P., LeMaoult, J., and Carosella, E.D. The dual role of HLA-G in cancer. J Immunol Res. 2014; : 359748
- Lin, A., Zhang, X., Xu, H.H., Xu, D.P., Ruan, Y.Y., and Yan, W.H. HLA-G expression is associated with metastasis and poor survival in the Balb/c nu/nu murine tumor model with ovarian cancer. Int J Cancer. 2012; 131: 150–157
- Li, B.L., Lin, A., Zhang, X.J. et al. Characterization of HLA-G expression in renal cell carcinoma. Tissue Antigens. 2009; 74: 213–221
- Bukur, J., Rebmann, V., Grosse-Wilde, H. et al. Functional role of human leukocyte antigen-G up-regulation in renal cell carcinoma. Cancer Res. 2003; 63: 4107–4111
- El-Chennawi, F.A., Auf, F.A., El-Diasty, A.M. et al. Expression of HLA-G in cancer bladder. Egypt J Immunol. 2005; 12: 57–64
- Gan, L.H., Huang, L.F., Zhang, X. et al. Tumor-specific upregulation of human leukocyte antigen-G expression in bladder transitional cell carcinoma. Hum Immunol. 2010; 71: 899–904
- Wang, L., Kang, F.-B., and Shan, B.-E. B7-H3-mediated tumor immunology: friend or foe?. Int J Cancer. 2014; 134: 2764–2771
- Ceeraz, S., Nowak, E.C., and Noelle, R.J. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013; 34: 556–563
- Jiang, J., Zhu, Y., Wu, C. et al. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010; 59: 1707–1714
- Krambeck, A.E., Thompson, R.H., Dong, H. et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006; 103: 10391–10396
- Tringler, B., Zhuo, S., Pilkington, G. et al. B7-H4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005; 11: 1842–1848
- Norde, W.J., Hobo, W., van der Voort, R., and Dolstra, H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012; 120: 728–736
- Ngiow, S.F., Teng, M.W.L., and Smyth, M.J. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011; 71: 6567–6571
- Liu, Y., Vlatkovic, L., Saeter, T. et al. Is the clinical malignant phenotype of prostate cancer a result of a highly proliferative immune-evasive B7-H3-expressing cell population?. Int J Urol. 2012; 19: 749–756
- Yuan, H., Wei, X., Zhang, G., Li, C., Zhang, X., and Hou, J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol. 2011; 186: 1093–1099
- Parker, A.S., Heckman, M.G., Sheinin, Y. et al. Evaluation of B7-H3 expression as a biomarker of biochemical recurrence after salvage radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2011; 79: 1343–1349
- Roth, T.J., Sheinin, Y., Lohse, C.M. et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007; 67: 7893–7900
- Brignone, C., Escudier, B., Grygar, C., Marcu, M., and Triebel, F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009; 15: 6225–6231
- Qin, X., Zhang, H., Ye, D., Dai, B., Zhu, Y., and Shi, G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther. 2013; 6: 1667–1673
- Crispen, P.L., Sheinin, Y., Roth, T.J. et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008; 14: 5150–5157
- Beck, K.E., Blansfield, J.A., Tran, K.Q. et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006; 24: 2283–2289
- Dolan, D.E. and Gupta, S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014; 21: 231–237
- Wolchok, J.D., Kluger, H., Callahan, M.K. et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013; 369: 122–133
- Curran, M.A., Montalvo, W., Yagita, H., and Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010; 107: 4275–4280
Gerelateerde artikelen
- CRISPR-Cas9 bewerkte T-cellen gericht op het intracellulaire immuuncontrolepunt CISH maakt patienten met gevorderde darmkanker alsnog gevoelig voor immuuntherapie met anti-PD medicijnen waar deze eerder ongevoelig voor waren of resistentie lieten zien. co
- DCVax-direct = immuuntherapie met dendritische cellen geeft hoopvolle resultaten bij 13 verschillende vormen van kanker met solide tumoren
- Solide tumoren: CRISPR-Cas9 gentherapie gericht op afwijkende DNA mutaties en gecombineerd met gemoduleerde T-cellen geeft hoopvolle resultaten bij drie patienten met vergevorderde kanker.
- Solide tumoren: Metronomische chemotherapie = dagelijks lage doses chemo naast immuuntherapie met nivolumab verbetert effectiviteit bij vormen van kanker met solide tumoren bij kinderen
- Solide tumoren: Tisotumab vedotin plus chemo geeft spectaculaire resultaten bij zwaarvoorbehandelde kankerpatienten met verschillende primaire vormen van kanker met solide tumoren.
- Solide tumoren: Interleukine-2 (IL-2) hoge dosis in medicijnproducerende bolletjes via kleine operatie ingebracht geneest muizen met gevorderde darmkanker en eierstokkanker binnen 6 dagen.
- Solide tumoren: Vesicular stomatitis virus (VSV-GP) een gemoduleerd oncolytisch virus geeft in combinatie met anti-PD medicijn ezabenlimab bij kankerpatienten met solide tumoren hoopvolle eerste resultaten
- Solide tumoren: Apceden, een gepersonaliseerd vaccin toegevoegd aan dendritische celtherapie geeft opvallend goede resultaten bij solide tumoren, waaronder ook prostaatkanker
- Solide tumoren: Hoge tumormutatiebelasting (TMB) als biomarker en prognose voor behandeling met immuuntherapie met anti-PD medicijnen is niet veel beter in uiteindelijk resultaat dan lage tumormutatiebelasting
- Solide tumoren: Lenvatinib + Pembrolizumab bij patiënten met inoperabele gevorderde nierkanker, baarmoederkanker, melanomen en andere gevorderde kanker met solide tumoren geeft uitstekende resultaten met meer dan de helft remissies van 50 procent of meer
- Solide tumoren: Vaccin tegen KRAS positief gemuteerde vormen van kanker - darmkankers en longkanker o.a. - wordt gecombineerd met trametinib een anti-PD medicijn in fase I studie na hoopvolle resultaten. copy 1
- Solide tumoren: pembrolizumab bij patiënten met vormen van uitgezaaide kanker, anders dan darmkanker, met hoge microsatellietinstabiliteit (MSI-H) en DNA-mismatch-reparatie-deficiënte (dMMR) geeft uitstekende en duurzame resultaten op overall overleving
- Solide tumoren: PD-L1 - blokkade met monoklonale anti lichamen - een vorm van immuuntherapie - zorgt bij verschillende vormen van kanker voor opmerkelijke resultaten
- Solide tumoren: Immuuntherapie op de PD-L1 expressie gericht met een zogeheten humaan monoklonaal antilichaam bekend onder de naam MPDL3280A zorgt al voor opmerkelijke resultaten in een fase I studie bij patiënten met solide tumoren
- Solide tumoren: Immuuntherapie met ipilimumab na gerichte stereotactische bestraling geeft uitstekende resultaten bij 26 ot 57 procent van100 zwaar voorbehandelde patienten met vergevorderde in lever en longen uitgezaaide kanker met solide tumoren
- Solide tumoren: Immuuntherapie alleen in vergelijking met immuuntherapie samen met chemotherapie bij kankerpatienten met solide tumoren geeft betere resultaten bij mannen dan bij vrouwen blijkt uit grote reviewstudie van 16 gerandomiseerde studies
- Solide tumoren: Immuuntherapie bij urologische vormen van kanker, blaaskanker, prostaatkanker en nierkanker is veelbelovend. Recente studies bewijzen langdurige remissies
- Solide tumoren: HPS - heat shock protein zorgt voor opmerkelijke resultaten bij allerlei vormen van kanker met solide tumoren. Vooral bij longkanker en darmkanker
- Solide tumoren: Immuuntherapie met nivolumab zorgt voor duurzame en sterk verbeterde overall overleving bij verschillende vormen van kanker, melanomen, longkanker en nierkanker
- Solide tumoren: Anti-PD medicijnen zoals nivolumab, Pembrolizumab en atezolizumab gegeven als immuuntherapie geven zeer goede resultaten bij verschillende vormen van kanker met solide tumoren, zelfs zonder Ligand-1 receptorstatus
- Solide tumoren: Vaccin gebaseerd op specifiek eiwit verbonden aan p53 gen en inspuiten van lichaamseigene dendritische cellen geeft veelbelovende resultaten in dierproeven bij niet-klein-cellige longkanker en borstkanker en hoofd en halstumoren.
- Solide tumoren: Kankerpatienten met solide tumoren met MSI-H = hoge microsatelliet instabiliteit en mismatch reparatie (dMMR) reageren uitstekend op immuuntherapie met pembrolizumab vooraf aan operatie met 65 tot 80 procent complete remissies




Plaats een reactie ...
Reageer op "Solide tumoren: Immuuntherapie bij urologische vormen van kanker, blaaskanker, prostaatkanker en nierkanker is veelbelovend. Recente studies bewijzen langdurige remissies"