23 oktober 2017: Bron: Frontier Oncology 2017; 7: 89. Published online 2017 May 22.
Een enkele keer krijg ik de vraag wat te doen tegen de bijwerkingen van bestraling van de mond en keel bij mond- en keelkanker. Enkele maanden geleden verscheen een studierapport dat richtlijnen geeft hoe met name orale mucositus = slijmvliesbeschadiging te behandelen en eventueel te voorkomen is. Mijn aanpak van destijds staat er niet bij maar wil ik toch hier wel noemen want het heeft mij veel geholpen. En ook anderen die ik de tips heb gegeven. zie verderop in dit artikel.
Hier een schaal van opbouw van opbouw en ernst van orale mucositis van de WHO: (tekst gaat verder onder dit plaatje)
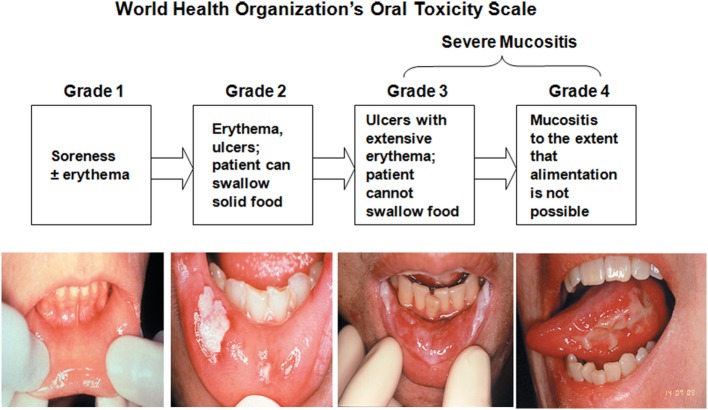
Deze maand ben ik 20 jaar kankervrij na mijn laatste operatie oktober 1997. Naast alle herinneringen staat me nog altijd bij hoeveel last ik had van de bijwerkingen van de bestralingen. Ik kon op een bepaald moment niet meer praten, niet meer normaal eten (had sondevoeding via neussonde nodig), mijn hele hals vertoonde open wonden die ook nog eens veel pijn deden en mijn hele mond zat vol wondjes, wat ze dus orale mucositis noemen. De slijmvliezen worden bijna altijd beschadigd door de bestraling.
De uiterlijke wonden in de hals heb ik nog tijdens de bestralingsperiode dichtgekregen via levertraanzalf (Jewcovitol is bij elke apotheek en drogist verkrijgbaar) die ik drie keer per dag ververste en met een verband bedekte. Binnen 5 dagen waren de wonden aan de buitenkant dicht. De verpleegkundigen op de afdeling radiotherapie in het academisch ziekenhuis Utrecht hadden dit nog nooit gezien en een van hun heeft ons wel eens verteld dit ook aan andere patienten door te hebben gegeven. Ook voor andere vormen van kanker kan levertraanzalf denk ik voorkomen dat er ernstige huidbeschadigingen ontstaan.
In de mond zelf heb ik de wondjes tijdens de bestralingsperiode niet weggekregen. Wel verminderde de pijn en na de bestraling kwam de speekselvorming zelfs weer grotendeels terug en mijn droge mond was na een jaar helemaal weg. Ik spoelde drie keer per dag met levertraan (Lovitran, verkrijgbaar in elke apotheek en drogist). Overigens worden door chemo ook vaak de slijmvliezen in de mond aangetast.

In de klinische praktijk worden andere middelen gebruikt om orale mucositis te voorkomen en bestrijden. Veel patienten, (80 tot 100 procent hebben op een of andere manier last van de bestraling van mond- en keel) hebben last van orale mucositis veroorzaakt door de bestraling - radiotherapie. Pijn in de mond komt voor bij 69% van de patiënten, dysfagie bij 56% van de patiënten, pijnstillers worden gebruikt door 53% van de patiënten, gewichtsverlies van 3-7 kg, door sondevoeding en ziekenhuisopname (ICU toelating) bij 15% van de patiënten. En wijziging of onderbreking van de behandeling gebeurt bij 11%-16% van de patiënten. Alle reden dus om te zoeken naar preventieve middelen die al deze problemen kunnen verminderen of beter nog te voorkomen.
In deze studie: Radiation-Induced Oral Mucositis worden onderstaand genoemde aanpak in deze grafiek besproken en vertaal ik maar niet. Lees daarvoor het studierapport. Maar m.i. is levertraan en levertraanzalf een heel goed en goedkoop alternatief om huidbeschadigingen en orale mucositis te voorkomen of te behandelen als het al is ontstaan. (Voor alle duidelijkheid ik/wij heb geen enkel belang met de producenten van levertraanzalf of levertraan, ik ken ze niet eens. Lees ook onze disclaimer)
Over low dose laser als manier om orale mucositis aan te pakken staat ook een en ander op onze website en in de literatuurlijsten.
Table 9
Multinational Association for Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO) Clinical Practice Guidelines for oral mucositis (3).
| Intervention/mode of administration | Purpose | Cancer treatment | Level of evidence |
|---|
| Recommendations in favor of an intervention (strong evidence supports effectiveness in the treatment setting listed) |
|
| Oral cryotherapy for 30 min |
Prevention of OM |
Patients receiving bolus 5-fluorouracil chemotherapy |
Level II |
|
| Recombinant human keratinocyte growth factor-1 (palifermin) at a dose of 60 µg/kg per day for 3 days prior to conditioning treatment and for 3 days after transplant |
Prevention of OM |
Patients receiving high-dose chemotherapy and TBI, followed by autologous stem cell transplantation, for a hematological malignancy |
Level II |
|
| Low-level laser therapy (wavelength at 650 nm, power of 40 mW, and each square centimeter treated with the required time to a tissue energy dose of 2 J/cm2) |
Prevention of OM |
Patients receiving HSCT conditioned with high-dose chemotherapy, with or without TBI |
Level II |
|
| Patient-controlled analgesia with morphine |
Pain reduction |
Patients undergoing HSCT |
Level II |
|
| Benzydamine mouthwash |
Prevention of OM |
Patients with HNC receiving moderate dose radiation therapy (up to 50 Gy), without concomitant chemotherapy |
Level II |
|
| Suggestions in favor of an intervention (weaker evidence supports effectiveness in the treatment setting listed) |
|
| Oral care protocols |
Prevention of OM |
All age groups and across all cancer treatment modalities |
Level III |
|
| Oral cryotherapy |
Prevention of OM |
Patients receiving high-dose melphalan, with or without TBI, as conditioning for HSCT |
Level III |
|
| Low-level laser therapy (wavelength around 632.8 nm) |
Prevention of OM |
Patients undergoing radiotherapy, without concomitant chemotherapy, for HNC |
Level III |
|
| Transdermal fentanyl |
Pain reduction |
Patients receiving conventional or high-dose chemotherapy, with or without TBI |
Level III |
|
| 2% morphine mouthwash |
Pain reduction |
Patients receiving chemoradiation for HNC |
Level III |
|
| 0.5% doxepin mouthwash |
Pain reduction |
All patients with OM-induced pain |
Level IV |
|
| Systemic zinc supplements administered orally |
Prevention of OM |
HNC patients receiving radiation therapy or chemoradiation |
Level III |
|
| Recommendations against interventions (strong evidence indicates lack of effectiveness in the treatment setting listed) |
|
| PTA (polymyxin, tobramycin, amphotericin B) and BCoG (bacitracin, clotrimazole, gentamicin) |
Prevention of OM |
Patients receiving radiation therapy for HNC |
Level II |
|
| Iseganan antimicrobial mouthwash |
Prevention of OM |
Patients receiving high-dose chemotherapy, with or without TBI, for HSCT or in patients receiving radiation therapy or concomitant chemoradiation for HNC |
Level II |
|
| Iseganan antimicrobial mouthwash |
Prevention of OM |
Patients receiving high-dose chemotherapy, with or without TBI, for HSCT or in patients receiving radiation therapy or concomitant chemoradiation for HNC |
Level II |
|
| Sucralfate mouthwash |
Prevention of OM |
Patients receiving chemotherapy for cancer (I), or inpatients receiving radiation therapy (I) or concomitant chemoradiation (II) for HNC |
Level I, II |
|
| Sucralfate mouthwash |
Treatment of OM |
Patients receiving chemotherapy for cancer (I), or in patients receiving radiation therapy (II) for HNC |
Level I, II |
|
| Intravenous glutamine |
Prevention of OM |
Patients receiving high-dose chemotherapy, with or without TBI, for HSCT |
Level II |
|
| Suggestions against interventions (weaker evidence indicates lack of effectiveness in the treatment setting listed) |
|
| Chlorhexidine mouthwash |
Prevention of OM |
Patients receiving radiation therapy for HNC |
Level III |
|
| Granulocyte-macrophage colony-stimulating factor mouthwash |
Prevention of OM |
Patients receiving high-dose chemotherapy, for autologous or allogeneic HSCT |
Level II |
|
| Misoprostol mouthwash |
Prevention of OM |
Patients receiving radiation therapy for HNC |
Level III |
|
| Systemic pentoxifylline, administered orally |
Prevention of OM |
Patients undergoing HSCT |
Level III |
|
| Systemic pilocarpine, administered orally |
Prevention of OM |
Patients receiving radiation therapy for head and neck cancer (III), or patients receiving high-dose chemotherapy, with or without TBI, for HSCT (II) |
Level II and III |
Het volledige studierapport: Radiation-Induced Oral Mucositis is gratis in te zien.
Hier het abstract van de studie met referentielijst:
RIOM treatment focuses on palliative measures and symptoms relief; e.g., pain management, nutritional support, good oral hygiene, and reduced oral microbial load. Interestingly, mesenchymal stromal cells therapy for RIOM shows promise for potential therapeutic and clinically relevant benefits.
Radiation-Induced Oral Mucositis
Abstract
Radiation-induced oral mucositis (RIOM) is a major dose-limiting toxicity in head and neck cancer patients. It is a normal tissue injury caused by radiation/radiotherapy (RT), which has marked adverse effects on patient quality of life and cancer therapy continuity. It is a challenge for radiation oncologists since it leads to cancer therapy interruption, poor local tumor control, and changes in dose fractionation. RIOM occurs in 100% of altered fractionation radiotherapy head and neck cancer patients. In the United Sates, its economic cost was estimated to reach 17,000.00 USD per patient with head and neck cancers. This review will discuss RIOM definition, epidemiology, impact and side effects, pathogenesis, scoring scales, diagnosis, differential diagnosis, prevention, and treatment.
Conclusion
Despite its high incidence, RIOM is a self-limited radiotherapy-induced normal tissue injury. It is a dose-limiting toxicity in most cases of head and neck cancer patients. However, in moderately to severely sick patients, it could be a lethal injury. Many preclinical and clinical studies have been conducted for the prevention and treatment of RIOM. Currently, there are numerous prevention and treatment strategies for RIOM. However, there is no single agent or management regimen that has been agreed upon between caregivers that significantly improves RIOM to a clinically relevant and satisfactory standard. Nevertheless, the current guidelines recommend good oral care, IMRT, radiation shields, palifermin, amifostine, and cryotherapy for RIOM prevention. RIOM treatment focuses on palliative measures and symptoms relief; e.g., pain management, nutritional support, good oral hygiene, and reduced oral microbial load. Interestingly, mesenchymal stromal cells therapy for RIOM shows promise for potential therapeutic and clinically relevant benefits. However, more studies are still needed to confirm such therapeutic potential.
Author Contributions
OM: conception and design, collection and/or assembly of data, review writing, and final approval of the review. NE: conception, design, and final approval of the review. TM: conception and design, financial support, and final approval of the review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. OM is an awardee of the Lady Davis Institute/Toronto-Dominion Bank studentship. This study was supported partially by Ride To Conquer Cancer (RTCC, Jewish General Hospital Foundation) and Fonds de Recherche du Quebec-Santé (FRQS) grants. English language editing was done by Jenny Warrington.
References
1.
Muanza TM, Cotrim AP, McAuliffe M, Sowers AL, Baum BJ, Cook JA, et al. Evaluation of radiation-induced oral mucositis by optical coherence tomography. Clin Cancer Res (2005) 11(14):5121–7.10.1158/1078-0432.CCR-05-0403 [PubMed] [Cross Ref]2.
Köstler WJ, Hejna M, Wenzel C, Zielinski CC. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin (2001) 51(5):290–315.10.3322/canjclin.51.5.290 [PubMed] [Cross Ref]3.
Al-Ansari S, Zecha JAEM, Barasch A, de Lange J, Rozema FR, Raber-Durlacher JE. Oral mucositis induced by anticancer therapies. Curr Oral Health Rep (2015) 2:202–11.10.1007/s40496-015-0069-4 [PMC free article] [PubMed] [Cross Ref]4.
Karthaus M, Rosenthal C, Ganser A. Prophylaxis and treatment of chemo- and radiotherapy-induced oral mucositis – are there new strategies? Bone Marrow Transplant (1999) 24(10):1095–108.10.1038/sj.bmt.1702024 [PubMed] [Cross Ref]5.
Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis—complicating the treatment of cancer. Neoplasia (2004) 6(5):423–31.10.1593/neo.04169 [PMC free article] [PubMed] [Cross Ref]6.
Rosenthal C, Karthaus M. [Current approaches in prevention and therapy of chemo- and radiotherapy-induced oral mucositis]. Wien Med Wochenschr (2001) 151(3–4):53–65. [PubMed]7.
Volpato LE, Silva TC, Oliveira TM, Sakai VT, Machado MA. Radiation therapy and chemotherapy-induced oral mucositis. Braz J Otorhinolaryngol (2007) 73(4):562–8.10.1016/S1808-8694(15)30110-5 [PubMed] [Cross Ref]8.
Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol (1998) 34(1):39–43.10.1016/S1368-8375(97)00053-5 [PubMed] [Cross Ref]9.
Feller L, Essop R, Wood NH, Khammissa RA, Chikte UM, Meyerov R, et al. Chemotherapy- and radiotherapy-induced oral mucositis: pathobiology, epidemiology and management. SADJ (2010) 65(8):372–4. [PubMed]10.
Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol (2004) 5:3–11. [PubMed]11.
Redding SW. Cancer therapy-related oral mucositis. J Dent Educ (2005) 69(8):919–29. [PubMed]12.
Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys (2007) 68(4):1110–20.10.1016/j.ijrobp.2007.01.053 [PubMed] [Cross Ref]13.
Luo DH, Hong MH, Guo L, Cao KJ, Deng MQ, Mo HY. [Analysis of oral mucositis risk factors during radiotherapy for nasopharyngeal carcinoma patients and establishment of a discriminant model]. Ai Zheng (2005) 24(7):850–4. [PubMed]14.
Chen SC, Lai YH, Huang BS, Lin CY, Fan KH, Chang JT. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur J Oncol Nurs (2015) 19(3):214–9.10.1016/j.ejon.2014.12.001 [PubMed] [Cross Ref]15.
Eilers J, Million R. Prevention and management of oral mucositis in patients with cancer. Semin Oncol Nurs (2007) 23:201–12.10.1016/j.soncn.2007.05.005 [PubMed] [Cross Ref]16.
Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol (2009) 45(12):1015–20.10.1016/j.oraloncology.2009.08.006 [PubMed] [Cross Ref]17.
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury. Cancer (2004) 100(9 Suppl):1995–2025.10.1002/cncr.20162 [PubMed] [Cross Ref]18.
Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells (2007) 25(11):2739–49.10.1634/stemcells.2007-0197 [PubMed] [Cross Ref]19.
Sonis ST. The pathobiology of mucositis. Nat Rev Cancer (2004) 4:277–84. [PubMed]20.
Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck (2004) 26(1):77–84.10.1002/hed.10326 [PubMed] [Cross Ref]21.
Etiz D, Orhan B, Demirüstü C, Ozdamar K, Cakmak A. Comparison of radiation-induced oral mucositis scoring systems. Tumori (2002) 88(5):379–84. [PubMed]22.
Riesenbeck D, Dorr W. Documentation of radiation-induced oral mucositis. Scoring systems. Strahlenther Onkol (1998) 174(Suppl 3):44–6. [PubMed]23.
Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH, Jr, Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer (1999) 85(10):2103–13.10.1002/(SICI)1097-0142(19990515)85:10<2103::AID-CNCR2>3.0.CO;2-0 [PubMed] [Cross Ref]24.
WCCNR. Assessing stomatitis: refinement of the Western Consortium for Cancer Nursing Research (WCCNR) stomatitis staging system. Can Oncol Nurs J (1998) 4:160–5. [PubMed]25.
Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys (2000) 47:13–47.10.1016/S0360-3016(99)00559-3 [PubMed] [Cross Ref]27.
McGuire DB, Peterson DE, Muller S, Owen DC, Slemmons MF, Schubert MM. The 20 item oral mucositis index: reliability and validity in bone marrow and stem cell transplant patients. Cancer Invest (2002) 20:893–903.10.1081/CNV-120005902 [PubMed] [Cross Ref]28.
Sonis ST, Oster G, Fuchs F, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol (2001) 19:2201–5.10.1200/JCO.2001.19.8.2201 [PubMed] [Cross Ref]29.
Parker L. Prevention and management of oral mucositis for an outpatient oncology setting. Okla Nurse (2005) 50:10–2. [PubMed]30.
Quinn B, Potting CM, Stone R, Blijlevens NM, Fliedner M, Margulies A, et al. Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer (2008) 44(1):61–72.10.1016/j.ejca.2007.09.014 [PubMed] [Cross Ref]31.
Uçüncü H, Ertekin MV, Yörük O, Sezen O, Ozkan A, Erdoğan F, et al. Vitamin E and l-carnitine, separately or in combination, in the prevention of radiation-induced oral mucositis and myelosuppression: a controlled study in a rat model. J Radiat Res (2006) 47(1):91–102.10.1269/jrr.47.91 [PubMed] [Cross Ref]32.
Schmidt W, Rainville LC, McEneff G, Sheehan D, Quinn B. A proteomic evaluation of the effects of the pharmaceuticals diclofenac and gemfibrozil on marine mussels (Mytilus spp.): evidence for chronic sublethal effects on stress-response proteins. Drug Test Anal (2014) 6(3):210–9.10.1002/dta.1463 [PubMed] [Cross Ref]33.
Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am (2008) 52(1):61–77,viii.10.1016/j.cden.2007.10.002 [PMC free article] [PubMed] [Cross Ref]34.
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer (2014) 120(10):1453–61.10.1002/cncr.28592 [PMC free article] [PubMed] [Cross Ref]35.
Lalla RV. The MASCC/ISOO mucositis guidelines update: introduction to the first set of articles. Support Care Cancer (2013) 21(1):301–2.10.1007/s00520-012-1660-z [PubMed] [Cross Ref]36.
Lalla RV, Ashbury FD. The MASCC/ISOO mucositis guidelines: dissemination and clinical impact. Support Care Cancer (2013) 21(11):3161–3.10.1007/s00520-013-1924-2 [PubMed] [Cross Ref]37.
Watanabe S, Suemaru K, Nakanishi M, Nakajima N, Tanaka M, Tanaka A, et al. Assessment of the hamster cheek pouch as a model for radiation-induced oral mucositis, and evaluation of the protective effects of keratinocyte growth factor using this model. Int J Radiat Biol (2014) 90(10):884–91.10.3109/09553002.2014.922716 [PubMed] [Cross Ref]38.
Zheng C, Cotrim AP, Sunshine AN, Sugito T, Liu L, Sowers A, et al. Prevention of radiation-induced oral mucositis after adenoviral vector-mediated transfer of the keratinocyte growth factor cDNA to mouse submandibular glands. Clin Cancer Res (2009) 15(14):4641–8.10.1158/1078-0432.CCR-09-0819 [PMC free article] [PubMed] [Cross Ref]39.
Kanuga S. Cryotherapy and keratinocyte growth factor may be beneficial in preventing oral mucositis in patients with cancer, and sucralfate is effective in reducing its severity. J Am Dent Assoc (2013) 144(8):928–9.10.14219/jada.archive.2013.0211 [PubMed] [Cross Ref]40.
Sonis ST. Efficacy of palifermin (keratinocyte growth factor-1) in the amelioration of oral mucositis. Core Evid (2009) 4:199–205.10.2147/CE.S5995 [PMC free article] [PubMed] [Cross Ref]41.
Tsirigotis P, Triantafyllou K, Girkas K, Giannopoulou V, Ioannidou E, Chondropoulos S, et al. Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: a video-capsule endoscopy study. Bone Marrow Transplant (2008) 42(5):337–43.10.1038/bmt.2008.168 [PubMed] [Cross Ref]42.
Blijlevens N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol (2007) 18(5):817–26.10.1093/annonc/mdl332 [PubMed] [Cross Ref]43.
Beaven AW, Shea TC. Recombinant human keratinocyte growth factor palifermin reduces oral mucositis and improves patient outcomes after stem cell transplant. Drugs Today (Barc) (2007) 43(7):461–73.10.1358/dot.2007.43.7.1119723 [PubMed] [Cross Ref]44.
Borges L, Rex KL, Chen JN, Wei P, Kaufman S, Scully S, et al. A protective role for keratinocyte growth factor in a murine model of chemotherapy and radiotherapy-induced mucositis. Int J Radiat Oncol Biol Phys (2006) 66(1):254–62.10.1016/j.ijrobp.2006.05.025 [PubMed] [Cross Ref]45.
Beaven AW, Shea TC. Palifermin: a keratinocyte growth factor that reduces oral mucositis after stem cell transplant for haematological malignancies. Expert Opin Pharmacother (2006) 7(16):2287–99.10.1517/14656566.7.16.2287 [PubMed] [Cross Ref]46.
Dorr W, Reichel S, Spekl K. Effects of keratinocyte growth factor (palifermin) administration protocols on oral mucositis (mouse) induced by fractionated irradiation. Radiother Oncol (2005) 75(1):99–105.10.1016/j.radonc.2004.12.006 [PubMed] [Cross Ref]47.
Dörr W, Bässler S, Reichel S, Spekl K. Reduction of radiochemotherapy-induced early oral mucositis by recombinant human keratinocyte growth factor (palifermin): experimental studies in mice. Int J Radiat Oncol Biol Phys (2005) 62(3):881–7.10.1016/j.ijrobp.2005.03.050 [PubMed] [Cross Ref]48.
Lee D, Jain VK. The use of recombinant human keratinocyte growth factor (palifermin) to ameliorate treatment-induced mucositis. Support Cancer Ther (2003) 1(1):20–2.10.1016/S1543-2912(13)60075-2 [PubMed] [Cross Ref]49.
Potten CS, Booth D, Cragg NJ, Tudor GL, O’Shea JA, Booth C, et al. Cell kinetic studies in the murine ventral tongue epithelium: mucositis induced by radiation and its protection by pretreatment with keratinocyte growth factor (KGF). Cell Prolif (2002) 35(Suppl 1):32–47.10.1046/j.1365-2184.35.s1.3.x [PubMed] [Cross Ref]50.
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, et al. The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol (2002) 50(1):53–8.10.1007/s00280-002-0460-4 [PubMed] [Cross Ref]51.
Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, et al. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif (2002) 35(Suppl 1):78–85.10.1046/j.1365-2184.35.s1.8.x [PubMed] [Cross Ref]52.
Dorr W, Spekl K, Farrell CL. Amelioration of acute oral mucositis by keratinocyte growth factor: fractionated irradiation. Int J Radiat Oncol Biol Phys (2002) 54(1):245–51.10.1016/S0360-3016(02)02918-8 [PubMed] [Cross Ref]53.
Dörr W, Noack R, Spekl K, Farrell CL. Modification of oral mucositis by keratinocyte growth factor: single radiation exposure. Int J Radiat Biol (2001) 77(3):341–7.10.1080/09553000010018873 [PubMed] [Cross Ref]54.
Bardet E, Martin L, Calais G, Alfonsi M, Feham NE, Tuchais C, et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the GORTEC2000-02 phase III randomized trial. J Clin Oncol (2011) 29(2):127–33.10.1200/JCO.2009.25.5638 [PubMed] [Cross Ref]55.
Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, Sauer R, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and-neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys (2005) 63(4):985–90.10.1016/j.ijrobp.2005.07.966 [PubMed] [Cross Ref]56.
Amrein PC, Clark JR, Supko JG, Fabian RL, Wang CC, Colevas AD, et al. Phase I trial and pharmacokinetics of escalating doses of paclitaxel and concurrent hyperfractionated radiotherapy with or without amifostine in patients with advanced head and neck carcinoma. Cancer (2005) 104(7):1418–27.10.1002/cncr.21312 [PubMed] [Cross Ref]57.
Komaki R, Lee JS, Milas L, Lee HK, Fossella FV, Herbst RS, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys (2004) 58(5):1369–77.10.1016/j.ijrobp.2003.10.005 [PubMed] [Cross Ref]58.
Karacetin D, Yücel B, Leblebicioğlu B, Aksakal O, Maral O, Incekara O. A randomized trial of amifostine as radioprotector in the radiotherapy of head and neck cancer. J BUON (2004) 9(1):23–6. [PubMed]59.
Athanassiou H, Antonadou D, Coliarakis N, Kouveli A, Synodinou M, Paraskevaidis M, et al. Protective effect of amifostine during fractionated radiotherapy in patients with pelvic carcinomas: results of a randomized trial. Int J Radiat Oncol Biol Phys (2003) 56(4):1154–60.10.1016/S0360-3016(03)00187-1 [PubMed] [Cross Ref]60.
Bardet E, Martin L, Calais G, Tuchais C, Bourhis J, Rhein B, et al. Preliminary data of the GORTEC 2000-02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Semin Oncol (2002) 29(6 Suppl 19):57–60.10.1053/sonc.2002.37348 [PubMed] [Cross Ref]61.
Li CJ, Wang SZ, Wang SY, Zhang YP. Assessment of the effect of local application of amifostine on acute radiation-induced oral mucositis in guinea pigs. J Radiat Res (2014) 55(5):847–54.10.1093/jrr/rru024 [PMC free article] [PubMed] [Cross Ref]62.
Praetorius NP, Mandal TK. Alternate delivery route for amifostine as a radio-/chemo-protecting agent. J Pharm Pharmacol (2008) 60(7):809–15.10.1211/jpp.60.7.0001 [PubMed] [Cross Ref]63.
Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist (2007) 12(6):738–47.10.1634/theoncologist.12-6-738 [PubMed] [Cross Ref]64.
Eisbruch A. Amifostine in the treatment of head and neck cancer: intravenous administration, subcutaneous administration, or none of the above. J Clin Oncol (2011) 29(2):119–21.10.1200/JCO.2010.31.5051 [PubMed] [Cross Ref]65.
Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One (2014) 9(5):e95968.10.1371/journal.pone.0095968 [PMC free article] [PubMed] [Cross Ref]66.
Keus R, Noach P, de Boer R, Lebesque J. The effect of customized beam shaping on normal tissue complications in radiation therapy of parotid gland tumors. Radiother Oncol (1991) 21(3):211–7.10.1016/0167-8140(91)90039-J [PubMed] [Cross Ref]67.
Kaanders JH, Fleming TJ, Ang KK, Maor MH, Peters LJ. Devices valuable in head and neck radiotherapy. Int J Radiat Oncol Biol Phys (1992) 23(3):639–45.10.1016/0360-3016(92)90023-B [PubMed] [Cross Ref]68.
Perch SJ, Machtay M, Markiewicz DA, Kligerman MM. Decreased acute toxicity by using midline mucosa-sparing blocks during radiation therapy for carcinoma of the oral cavity, oropharynx, and nasopharynx. Radiology (1995) 197(3):863–6.10.1148/radiology.197.3.7480771 [PubMed] [Cross Ref]69.
Kouloulias V, Thalassinou S, Platoni K, Zygogianni A, Kouvaris J, Antypas C, et al. The treatment outcome and radiation-induced toxicity for patients with head and neck carcinoma in the IMRT era: a systematic review with dosimetric and clinical parameters. Biomed Res Int (2013) 2013:401261.10.1155/2013/401261 [PMC free article] [PubMed] [Cross Ref]70.
Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M, et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer (1999) 7(4):244–52.10.1007/s005200050256 [PubMed] [Cross Ref]71.
Barber C, Powell R, Ellis A, Hewett J. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: a preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support Care Cancer (2007) 15(4):427–40.10.1007/s00520-006-0171-1 [PubMed] [Cross Ref]72.
Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer (1990) 66(1):62–8.10.1002/1097-0142(19900701)66:1<62::AID-CNCR2820660113>3.0.CO;2-E [PubMed] [Cross Ref]73.
Huang EY, Leung SW, Wang CJ, Chen HC, Sun LM, Fang FM, et al. Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys (2000) 46(3):535–9.10.1016/S0360-3016(99)00402-2 [PubMed] [Cross Ref]74.
Peterson DE, Jones JB, Petit RG, II. Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer (2007) 109(2):322–31.10.1002/cncr.22384 [PubMed] [Cross Ref]75.
Grumetto L, Del Prete A, Ortosecco G, Barbato F, Del Prete S, Borrelli A, et al. Study on the protective effect of a new manganese superoxide dismutase on the microvilli of rabbit eyes exposed to UV radiation. Biomed Res Int (2015) 2015:973197.10.1155/2015/973197 [PMC free article] [PubMed] [Cross Ref]76.
Eldridge A, Fan M, Woloschak G, Grdina DJ, Chromy BA, Li JJ. Manganese superoxide dismutase interacts with a large scale of cellular and mitochondrial proteins in low-dose radiation-induced adaptive radioprotection. Free Radic Biol Med (2012) 53(10):1838–47.10.1016/j.freeradbiomed.2012.08.589 [PMC free article] [PubMed] [Cross Ref]77.
Rajagopalan MS, Stone B, Rwigema JC, Salimi U, Epperly MW, Goff J, et al. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total-body and thoracic radiation sensitivity of NOS1−/− mice. Radiat Res (2010) 174(3):297–312.10.1667/RR2019.1 [PMC free article] [PubMed] [Cross Ref]78.
Holley AK, Xu Y, St Clair DK, St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Ann N Y Acad Sci (2010) 1201:129–36.10.1111/j.1749-6632.2010.05613.x [PMC free article] [PubMed] [Cross Ref]79.
dos Santos Montagner GF, Sagrillo M, Machado MM, Almeida RC, Mostardeiro CP, Duarte MM, et al. Toxicological effects of ultraviolet radiation on lymphocyte cells with different manganese superoxide dismutase Ala16Val polymorphism genotypes. Toxicol In Vitro (2010) 24(5):1410–6.10.1016/j.tiv.2010.04.010 [PubMed] [Cross Ref]80.
Holley AK, St Clair DK. Preventing Dr. Jekyll from becoming Mr. Hyde: is manganese superoxide dismutase the key to prevent radiation-induced neoplastic transformation? Cancer Biol Ther (2009) 8(20):1972–3.10.4161/cbt.8.20.9941 [PMC free article] [PubMed] [Cross Ref]81.
Josson S, Xu Y, Fang F, Dhar SK, St Clair DK, St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene (2006) 25(10):1554–9.10.1038/sj.onc.1209186 [PMC free article] [PubMed] [Cross Ref]82.
Guo HL, Zhao HW, Xu ZF, Ma H, Song XL, Guan J, et al. [Manganese superoxide dismutase gene transfection of mouse small intestinal epithelial cells protects them from radiation injury]. Zhonghua Zhong Liu Za Zhi (2005) 27(11):672–5. [PubMed]83.
Guo HL, Wolfe D, Epperly MW, Huang S, Liu K, Glorioso JC, et al. Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury. J Gastrointest Surg (2003) 7(2):229–35; discussion 235–6. [PubMed]84.
Guo H, Seixas-Silva JA, Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res (2003) 159(3):361–70.10.1667/0033-7587(2003)159[0361:PORIOC]2.0.CO;2 [PubMed] [Cross Ref]85.
Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, et al. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol (2003) 23(7):2362–78.10.1128/MCB.23.7.2362-2378.2003 [PMC free article] [PubMed] [Cross Ref]86.
Epperly MW, Bernarding M, Gretton J, Jefferson M, Nie S, Greenberger JS. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-alpha, IL-3 withdrawal, and ionizing radiation. Exp Hematol (2003) 31(6):465–74.10.1016/S0301-472X(03)00041-9 [PubMed] [Cross Ref]87.
Epperly MW, Sikora CA, DeFilippi SJ, Gretton JA, Zhan Q, Kufe DW, et al. Manganese superoxide dismutase (SOD2) inhibits radiation-induced apoptosis by stabilization of the mitochondrial membrane. Radiat Res (2002) 157(5):568–77.10.1667/0033-7587(2002)157[0568:MSDSIR]2.0.CO;2 [PubMed] [Cross Ref]88.
Motoori S, Majima HJ, Ebara M, Kato H, Hirai F, Kakinuma S, et al. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res (2001) 61(14):5382–8. [PubMed]89.
Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, et al. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated radiation. Int J Cancer (2001) 96(4):221–31.10.1002/ijc.1023 [PubMed] [Cross Ref]90.
Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, et al. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res (2001) 155(1 Pt 1):2–14.10.1667/0033-7587(2001)155[0002:MORICE]2.0.CO;2 [PubMed] [Cross Ref]91.
Kuninaka S, Ichinose Y, Koja K, Toh Y. Suppression of manganese superoxide dismutase augments sensitivity to radiation, hyperthermia and doxorubicin in colon cancer cell lines by inducing apoptosis. Br J Cancer (2000) 83(7):928–34.10.1054/bjoc.2000.1367 [PMC free article] [PubMed] [Cross Ref]92.
Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiat Res (2000) 154(4):365–74.10.1667/0033-7587(2000)154[0365:DPRROM]2.0.CO;2 [PubMed] [Cross Ref]93.
Sasaki H, Akamatsu H, Horio T. Effects of a single exposure to UVB radiation on the activities and protein levels of copper-zinc and manganese superoxide dismutase in cultured human keratinocytes. Photochem Photobiol (1997) 65(4):707–13.10.1111/j.1751-1097.1997.tb01914.x [PubMed] [Cross Ref]94.
Otero G, Avila MA, Emfietzoglou D, Clerch LB, Massaro D, Notario V. Increased manganese superoxide dismutase activity, protein, and mRNA levels and concurrent induction of tumor necrosis factor alpha in radiation-initiated Syrian hamster cells. Mol Carcinog (1996) 17(4):175–80.10.1002/(SICI)1098-2744(199612)17:4<175::AID-MC1>3.0.CO;2-D [PubMed] [Cross Ref]95.
Nakano T, Oka K, Taniguchi N. Manganese superoxide dismutase expression correlates with p53 status and local recurrence of cervical carcinoma treated with radiation therapy. Cancer Res (1996) 56(12):2771–5. [PubMed]96.
Urano M, Kuroda M, Reynolds R, Oberley TD, St Clair DK. Expression of manganese superoxide dismutase reduces tumor control radiation dose: gene-radiotherapy. Cancer Res (1995) 55(12):2490–3. [PubMed]97.
Lin PS, Ho KC, Sung SJ, Tsai S. Cytotoxicity and manganese superoxide dismutase induction by tumor necrosis factor-alpha and ionizing radiation in MCF-7 human breast carcinoma cells. Lymphokine Cytokine Res (1993) 12(5):303–8. [PubMed]98.
Hirose K, Longo DL, Oppenheim JJ, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J (1993) 7(2):361–8. [PubMed]99.
LeVeque FG, Parzuchowski JB, Farinacci GC, Redding SW, Rodu B, Johnson JT, et al. Clinical evaluation of MGI 209, an anesthetic, film-forming agent for relief from painful oral ulcers associated with chemotherapy. J Clin Oncol (1992) 10(12):1963–8.10.1200/JCO.1992.10.12.1963 [PubMed] [Cross Ref]100.
Barker G, Loftus L, Cuddy P, Barker B. The effects of sucralfate suspension and diphenhydramine syrup plus kaolin-pectin on radiotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol (1991) 71(3):288–93.10.1016/0030-4220(91)90301-R [PubMed] [Cross Ref]101.
Su YX, Benedek GA, Sieg P, Liao GQ, Dendorfer A, Meller B, et al. Radioprotective effect of lidocaine on neurotransmitter agonist-induced secretion in irradiated salivary glands. PLoS One (2013) 8(3):e60256.10.1371/journal.pone.0060256 [PMC free article] [PubMed] [Cross Ref]102.
Carnel SB, Blakeslee DB, Oswald SG, Barnes M. Treatment of radiation- and chemotherapy-induced stomatitis. Otolaryngol Head Neck Surg (1990) 102(4):326–30.10.1177/019459989010200404 [PubMed] [Cross Ref]103.
Rodu B, Russell CM, Ray KL. Treatment of oral ulcers with hydroxypropylcellulose film (Zilactin). Compendium (1988) 9(5):420–2. [PubMed]104.
Sung L, Robinson P, Treister N, Baggott T, Gibson P, Tissing W, et al. Guideline for the prevention of oral and oropharyngeal mucositis in children receiving treatment for cancer or undergoing haematopoietic stem cell transplantation. BMJ Support Palliat Care (2017) 7(1):7–16.10.1136/bmjspcare-2014-000804 [PMC free article] [PubMed] [Cross Ref]105.
Miller MM, Donald DV, Hagemann TM. Prevention and treatment of oral mucositis in children with cancer. J Pediatr Pharmacol Ther (2012) 17(4):340–50.10.5863/1551-6776-17.4.340 [PMC free article] [PubMed] [Cross Ref]106.
Ps SK, Balan A, Sankar A, Bose T. Radiation induced oral mucositis. Indian J Palliat Care (2009) 15(2):95–102.10.4103/0973-1075.58452 [PMC free article] [PubMed] [Cross Ref]107.
Sarvizadeh M, Hemati S, Meidani M, Ashouri M, Roayaei M, Shahsanai A. Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv Biomed Res (2015) 4:44.10.4103/2277-9175.151254 [PMC free article] [PubMed] [Cross Ref]108.
Vayne-Bossert P, Escher M, de Vautibault CG, Dulguerov P, Allal A, Desmeules J, et al. Effect of topical morphine (mouthwash) on oral pain due to chemotherapy- and/or radiotherapy-induced mucositis: a randomized double-blinded study. J Palliat Med (2010) 13(2):125–8.10.1089/jpm.2009.0195 [PubMed] [Cross Ref]109.
Rothwell BR, Spektor WS. Palliation of radiation-related mucositis. Spec Care Dentist (1990) 10(1):21–5.10.1111/j.1754-4505.1990.tb01082.x [PubMed] [Cross Ref]110.
Murata Y, Kofuji K, Nishida N, Kamaguchi R. Development of film dosage form containing allopurinol for prevention and treatment of oral mucositis. ISRN Pharm (2012) 2012:764510.10.5402/2012/764510 [PMC free article] [PubMed] [Cross Ref]111.
Tomoda K, Asahiyama M, Ohtsuki E, Nakajima T, Terada H, Kanebako M, et al. Preparation and properties of carrageenan microspheres containing allopurinol and local anesthetic agents for the treatment of oral mucositis. Colloids Surf B Biointerfaces (2009) 71(1):27–35.10.1016/j.colsurfb.2009.01.003 [PubMed] [Cross Ref]112.
Kitagawa J, Nasu M, Okumura H, Shibata A, Makino K, Terada H, et al. Allopurinol gel mitigates radiation-induced mucositis and dermatitis. J Radiat Res (2008) 49(1):49–54.10.1269/jrr.07038 [PubMed] [Cross Ref]113.
Loprinzi CL, Burnham N. Allopurinol mouthwash as prophylactic therapy for 5-fluorouracil-induced mucositis. Eur J Surg Oncol (1989) 15(3):297. [PubMed]114.
Renck D, Santos AA, Jr, Machado P, Petersen GO, Lopes TG, Santos DS, et al. Human uridine phosphorylase-1 inhibitors: a new approach to ameliorate 5-fluorouracil-induced intestinal mucositis. Invest New Drugs (2014) 32(6):1301–7.10.1007/s10637-014-0135-0 [PubMed] [Cross Ref]115.
Panahi Y, Ala S, Saeedi M, Okhovatian A, Bazzaz N, Naghizadeh MM. Allopurinol mouth rinse for prophylaxis of fluorouracil-induced mucositis. Eur J Cancer Care (Engl) (2010) 19(3):308–12.10.1111/j.1365-2354.2008.01042.x [PubMed] [Cross Ref]116.
Seiter K, Kemeny N, Martin D, Schneider A, Williams L, Colofiore J, et al. Uridine allows dose escalation of 5-fluorouracil when given with N-phosphonacetyl-l-aspartate, methotrexate, and leucovorin. Cancer (1993) 71(5):1875–81.10.1002/1097-0142(19930301)71:5<1875::AID-CNCR2820710526>3.0.CO;2-9 [PubMed] [Cross Ref]117.
Foote RL, Loprinzi CL, Frank AR, O’Fallon JR, Gulavita S, Tewfik HH, et al. Randomized trial of a chlorhexidine mouthwash for alleviation of radiation-induced mucositis. J Clin Oncol (1994) 12(12):2630–3.10.1200/JCO.1994.12.12.2630 [PubMed] [Cross Ref]118.
Roopashri G, Jayanthi K, Guruprasad R. Efficacy of benzydamine hydrochloride, chlorhexidine, and povidone iodine in the treatment of oral mucositis among patients undergoing radiotherapy in head and neck malignancies: a drug trail. Contemp Clin Dent (2011) 2(1):8–12.10.4103/0976-237X.79292 [PMC free article] [PubMed] [Cross Ref]119.
Dodd MJ, Larson PJ, Dibble SL, Miaskowski C, Greenspan D, MacPhail L, et al. Randomized clinical trial of chlorhexidine versus placebo for prevention of oral mucositis in patients receiving chemotherapy. Oncol Nurs Forum (1996) 23(6):921–7. [PubMed]120.
de Boer-Dennert MM, Batchelor D. [“Randomized clinical trial of chlorhexidine versus placebo for prevention of oral mucositis in patients receiving chemotherapy”. Marylin J. Dodd et al. Report of discussion of this article in the IKA Nursing Research Utilization Board]. Oncologica (1997) 14(3):16–8. [PubMed]121.
Davies AN, Singer J. A comparison of artificial saliva and pilocarpine in radiation-induced xerostomia. J Laryngol Otol (1994) 108(8):663–5.10.1017/S0022215100127768 [PubMed] [Cross Ref]122.
Dos Reis PE, Ciol MA, de Melo NS, Figueiredo PT, Leite AF, Manzi Nde M. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: a pilot study. Support Care Cancer (2016) 24(10):4393–8.10.1007/s00520-016-3279-y [PubMed] [Cross Ref]123.
Curra M, Martins MA, Lauxen IS, Pellicioli AC, Sant’Ana Filho M, Pavesi VC, et al. Effect of topical chamomile on immunohistochemical levels of IL-1beta and TNF-alpha in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother Pharmacol (2013) 71(2):293–9.10.1007/s00280-012-2013-9 [PubMed] [Cross Ref]124.
Pavesi VC, Lopez TC, Martins MA, Sant’Ana Filho M, Bussadori SK, Fernandes KP, et al. Healing action of topical chamomile on 5-fluoracil induced oral mucositis in hamster. Support Care Cancer (2011) 19(5):639–46.10.1007/s00520-010-0875-0 [PubMed] [Cross Ref]125.
Mazokopakis EE, Vrentzos GE, Papadakis JA, Babalis DE, Ganotakis ES. Wild chamomile (Matricaria recutita L.) mouthwashes in methotrexate-induced oral mucositis. Phytomedicine (2005) 12(1–2):25–7.10.1016/j.phymed.2003.11.003 [PubMed] [Cross Ref]126.
Fidler P, Loprinzi CL, O’Fallon JR, Leitch JM, Lee JK, Hayes DL, et al. Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer (1996) 77(3):522–5.10.1002/(SICI)1097-0142(19960201)77:3<522::AID-CNCR14>3.0.CO;2-6 [PubMed] [Cross Ref]127.
Van den Wyngaert T. Topical honey application to reduce radiation-induced oral mucositis: a therapy too sweet to ignore? J Evid Based Dent Pract (2012) 12(4):203–5.10.1016/j.jebdp.2012.09.011 [PubMed] [Cross Ref]128.
Song JJ, Twumasi-Ankrah P, Salcido R. Systematic review and meta-analysis on the use of honey to protect from the effects of radiation-induced oral mucositis. Adv Skin Wound Care (2012) 25(1):23–8.10.1097/01.ASW.0000410687.14363.a3 [PubMed] [Cross Ref]129.
Khanal B, Baliga M, Uppal N. Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. Int J Oral Maxillofac Surg (2010) 39(12):1181–5.10.1016/j.ijom.2010.05.014 [PubMed] [Cross Ref]130.
Bardy J, Molassiotis A, Ryder WD, Mais K, Sykes A, Yap B, et al. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br J Oral Maxillofac Surg (2012) 50(3):221–6.10.1016/j.bjoms.2011.03.005 [PubMed] [Cross Ref]131.
Santos-Silva AR, Rosa GB, Eduardo CP, Dias RB, Brandao TB. Increased risk for radiation-related caries in cancer patients using topical honey for the prevention of oral mucositis. Int J Oral Maxillofac Surg (2011) 40(11):1335–6; author reply 1235.10.1016/j.ijom.2011.05.006 [PubMed] [Cross Ref]132.
Arora H, Pai KM, Maiya A, Vidyasagar MS, Rajeev A. Efficacy of He-Ne laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2008) 105(2):180–6, 186.e1.10.1016/j.tripleo.2007.07.043 [PubMed] [Cross Ref]133.
Hawley P, Hovan A, McGahan CE, Saunders D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer (2014) 22(3):751–61.10.1007/s00520-013-2031-0 [PubMed] [Cross Ref]134.
Ala S, Saeedi M, Janbabai G, Ganji R, Azhdari E, Shiva A. Efficacy of sucralfate mouth wash in prevention of 5-fluorouracil induced oral mucositis: a prospective, randomized, double-blind, controlled trial. Nutr Cancer (2016) 68(3):456–63.10.1080/01635581.2016.1153666 [PubMed] [Cross Ref]135.
Nottage M, McLachlan SA, Brittain MA, Oza A, Hedley D, Feld R, et al. Sucralfate mouthwash for prevention and treatment of 5-fluorouracil-induced mucositis: a randomized, placebo-controlled trial. Support Care Cancer (2003) 11(1):41–7.10.1007/s00520-002-0378-8 [PubMed] [Cross Ref]136.
Dodd MJ, Miaskowski C, Greenspan D, MacPhail L, Shih AS, Shiba G, et al. Radiation-induced mucositis: a randomized clinical trial of micronized sucralfate versus salt & soda mouthwashes. Cancer Invest (2003) 21(1):21–33.10.1081/CNV-120016400 [PubMed] [Cross Ref]137.
Saarilahti K, Kajanti M, Joensuu T, Kouri M, Joensuu H. Comparison of granulocyte-macrophage colony-stimulating factor and sucralfate mouthwashes in the prevention of radiation-induced mucositis: a double-blind prospective randomized phase III study. Int J Radiat Oncol Biol Phys (2002) 54(2):479–85.10.1016/S0360-3016(02)02935-8 [PubMed] [Cross Ref]138.
Kilic D, Akcali Z. Comment on: granulocyte macrophage-colony stimulating factor (GM-CSF) and sucralfate in prevention of radiation-induced mucositis: a prospective randomized study. Int J Radiat Oncol Biol Phys (2001) 50(5):1373–4.10.1016/S0360-3016(01)01587-5 [PubMed] [Cross Ref]139.
Castagna L, Benhamou E, Pedraza E, Luboinski M, Forni M, Brandes I, et al. Prevention of mucositis in bone marrow transplantation: a double blind randomised controlled trial of sucralfate. Ann Oncol (2001) 12(7):953–5.10.1023/A:1011119721267 [PubMed] [Cross Ref]140.
Makkonen TA, Minn H, Jekunen A, Vilja P, Tuominen J, Joensuu H. Granulocyte macrophage-colony stimulating factor (GM-CSF) and sucralfate in prevention of radiation-induced mucositis: a prospective randomized study. Int J Radiat Oncol Biol Phys (2000) 46(3):525–34.10.1016/S0360-3016(99)00452-6 [PubMed] [Cross Ref]141.
Etiz D, Erkal HS, Serin M, Küçük B, Hepari A, Elhan AH, et al. Clinical and histopathological evaluation of sucralfate in prevention of oral mucositis induced by radiation therapy in patients with head and neck malignancies. Oral Oncol (2000) 36(1):116–20.10.1016/S1368-8375(99)00075-5 [PubMed] [Cross Ref]142.
Cengiz M, Ozyar E, Oztürk D, Akyol F, Atahan IL, Hayran M. Sucralfate in the prevention of radiation-induced oral mucositis. J Clin Gastroenterol (1999) 28(1):40–3.10.1097/00004836-199901000-00009 [PubMed] [Cross Ref]143.
Sur RK. Sucralfate in radiation-induced mucositis. S Afr Med J (1997) 87(3):337–8. [PubMed]144.
Meredith R, Salter M, Kim R, Spencer S, Weppelmann B, Rodu B, et al. Sucralfate for radiation mucositis: results of a double-blind randomized trial. Int J Radiat Oncol Biol Phys (1997) 37(2):275–9.10.1016/S0360-3016(96)00531-7 [PubMed] [Cross Ref]145.
Franzén L, Henriksson R, Littbrand B, Zackrisson B. Effects of sucralfate on mucositis during and following radiotherapy of malignancies in the head and neck region. A double-blind placebo-controlled study. Acta Oncol (1995) 34(2):219–23.10.3109/02841869509093959 [PubMed] [Cross Ref]146.
Allison RR, Vongtama V, Vaughan J, Shin KH. Symptomatic acute mucositis can be minimized or prophylaxed by the combination of sucralfate and fluconazole. Cancer Invest (1995) 13(1):16–22.10.3109/07357909509024890 [PubMed] [Cross Ref]147.
Makkonen TA, Boström P, Vilja P, Joensuu H. Sucralfate mouth washing in the prevention of radiation-induced mucositis: a placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys (1994) 30(1):177–82.10.1016/0360-3016(94)90533-9 [PubMed] [Cross Ref]148.
Epstein JB, Wong FL. The efficacy of sucralfate suspension in the prevention of oral mucositis due to radiation therapy. Int J Radiat Oncol Biol Phys (1994) 28(3):693–8.10.1016/0360-3016(94)90195-3 [PubMed] [Cross Ref]149.
Shenep JL, Kalwinsky DK, Hutson PR, George SL, Dodge RK, Blankenship KR, et al. Efficacy of oral sucralfate suspension in prevention and treatment of chemotherapy-induced mucositis. J Pediatr (1988) 113(4):758–63.10.1016/S0022-3476(88)80397-4 [PubMed] [Cross Ref]150.
Theodore C, Thurninger O, Hermitte H. [Radiation-induced mucositis: a new indication of sucralfate?]. Gastroenterol Clin Biol (1987) 11(4):345. [PubMed]151.
Solomon MA. Oral sucralfate suspension for mucositis. N Engl J Med (1986) 315(7):459–60.10.1056/NEJM198608143150717 [PubMed] [Cross Ref]152.
High KP, Legault C, Sinclair JA, Cruz J, Hill K, Hurd DD. Low plasma concentrations of retinol and alpha-tocopherol in hematopoietic stem cell transplant recipients: the effect of mucositis and the risk of infection. Am J Clin Nutr (2002) 76(6):1358–66. [PubMed]153.
Cohen G, Elad S, Or R, Galili D, Garfunkel AA. The use of tretinoin as oral mucositis prophylaxis in bone marrow transplantation patients: a preliminary study. Oral Dis (1997) 3(4):243–6.10.1111/j.1601-0825.1997.tb00049.x [PubMed] [Cross Ref]154.
Ferreira PR, Fleck JF, Diehl A, Barletta D, Braga-Filho A, Barletta A, et al. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: a double-blind randomized trial. Head Neck (2004) 26(4):313–21.10.1002/hed.10382 [PubMed] [Cross Ref]155.
Oshitani T, Okada K, Kushima T, Suematsu T, Obayashi K, Hirata Y, et al. [Clinical evaluation of sodium alginate on oral mucositis associated with radiotherapy]. Nihon Gan Chiryo Gakkai Shi (1990) 25(6):1129–37. [PubMed]156.
Epstein JB, Stevenson-Moore P, Jackson S, Mohamed JH, Spinelli JJ. Prevention of oral mucositis in radiation therapy: a controlled study with benzydamine hydrochloride rinse. Int J Radiat Oncol Biol Phys (1989) 16(6):1571–5.10.1016/0360-3016(89)90964-4 [PubMed] [Cross Ref]157.
Epstein JB, Stevenson-Moore P. Benzydamine hydrochloride in prevention and management of pain in oral mucositis associated with radiation therapy. Oral Surg Oral Med Oral Pathol (1986) 62(2):145–8.10.1016/0030-4220(86)90035-6 [PubMed] [Cross Ref]158.
Rahn R, Adamietz IA, Boettcher HD, Schaefer V, Reimer K, Fleischer W. Povidone-iodine to prevent mucositis in patients during antineoplastic radiochemotherapy. Dermatology (1997) 195(Suppl 2):57–61.10.1159/000246032 [PubMed] [Cross Ref]159.
Adamietz IA, Rahn R, Böttcher HD, Schäfer V, Reimer K, Fleischer W. Prophylaxis with povidone-iodine against induction of oral mucositis by radiochemotherapy. Support Care Cancer (1998) 6(4):373–7.10.1007/s005200050179 [PubMed] [Cross Ref]160.
Vokurka S, Bystricka E, Koza V, Scudlova J, Pavlicova V, Valentova D, et al. The comparative effects of povidone-iodine and normal saline mouthwashes on oral mucositis in patients after high-dose chemotherapy and APBSCT – results of a randomized multicentre study. Support Care Cancer (2005) 13(7):554–8.10.1007/s00520-005-0792-9 [PubMed] [Cross Ref]161.
Berger A, Henderson M, Nadoolman W, Duffy V, Cooper D, Saberski L, et al. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J Pain Symptom Manage (1995) 10(3):243–8.10.1016/0885-3924(94)00130-D [PubMed] [Cross Ref]162.
Lalla RV, Pilbeam CC, Walsh SJ, Sonis ST, Keefe DM, Peterson DE. Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Support Care Cancer (2010) 18(1):95–103.10.1007/s00520-009-0635-1 [PMC free article] [PubMed] [Cross Ref]163.
Lopes NN, Plapler H, Chavantes MC, Lalla RV, Yoshimura EM, Alves MT. Cyclooxygenase-2 and vascular endothelial growth factor expression in 5-fluorouracil-induced oral mucositis in hamsters: evaluation of two low-intensity laser protocols. Support Care Cancer (2009) 17(11):1409–15.10.1007/s00520-009-0603-9 [PubMed] [Cross Ref]164.
Sonis ST, O’Donnell KE, Popat R, Bragdon C, Phelan S, Cocks D, et al. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol (2004) 40(2):170–6.10.1016/S1368-8375(03)00148-9 [PubMed] [Cross Ref]165.
Pillsbury HC, III, Webster WP, Rosenman J. Prostaglandin inhibitor and radiotherapy in advanced head and neck cancers. Arch Otolaryngol Head Neck Surg (1986) 112(5):552–3.10.1001/archotol.1986.03780050076013 [PubMed] [Cross Ref]166.
Kono T, Kaneko A, Matsumoto C, Miyagi C, Ohbuchi K, Mizuhara Y, et al. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integr Cancer Ther (2014) 13(5):435–45.10.1177/1534735413520035 [PubMed] [Cross Ref]167.
Hanson WR, Marks JE, Reddy SP, Simon S, Mihalo WE, Tova Y. Protection from radiation-induced oral mucositis by a mouth rinse containing the prostaglandin E1 analog, misoprostol: a placebo controlled double blind clinical trial. Adv Exp Med Biol (1997) 400B:811–8. [PubMed]168.
Maltoni M, Sansoni E, Derni S, Milandri C, Martini F, Nanni O, et al. Topical prostaglandin E2 and chemo- and radio-induced oral mucositis. Oncol Rep (1996) 3(1):205–8. [PubMed]169.
Hanson WR, Marks JE, Reddy SP, Simon S, Mihalo WE, Tova Y. Protection from radiation-induced oral mucositis by misoprostol, a prostaglandin E(1) analog: a placebo-controlled, double-blind clinical trial. Am J Ther (1995) 2(11):850–7.10.1097/00045391-199511000-00005 [PubMed] [Cross Ref]170.
Labar B, Mrsić M, Pavletić Z, Bogdanić V, Nemet D, Aurer I, et al. Prostaglandin E2 for prophylaxis of oral mucositis following BMT. Bone Marrow Transplant (1993) 11(5):379–82. [PubMed]171.
Matejka M, Nell A, Kment G, Schein A, Leukauf M, Porteder H, et al. Local benefit of prostaglandin E2 in radiochemotherapy-induced oral mucositis. Br J Oral Maxillofac Surg (1990) 28(2):89–91.10.1016/0266-4356(90)90128-8 [PubMed] [Cross Ref]172.
Pretnar J, Glazar D, Mlakar U, Modic M. Prostaglandin E2 in the treatment of oral mucositis due to radiochemotherapy in patients with haematological malignancies. Bone Marrow Transplant (1989) 4(Suppl 3):106. [PubMed]173.
He D, Behar S, Roberts JE, Lim HW. The effect of l-cysteine and N-acetylcysteine on porphyrin/heme biosynthetic pathway in cells treated with 5-aminolevulinic acid and exposed to radiation. Photodermatol Photoimmunol Photomed (1996) 12(5):194–9.10.1111/j.1600-0781.1996.tb00199.x [PubMed] [Cross Ref]174.
Baier JE, Neumann HA, Moeller T, Kissler M, Borchardt D, Ricken D. [Radiation protection through cytokine release by N-acetylcysteine]. Strahlenther Onkol (1996) 172(2):91–8. [PubMed]175.
Ozgur E, Sahin D, Tomruk A, Guler G, Sepici Dinçel A, Altan N, et al. The effects of N-acetylcysteine and epigallocatechin-3-gallate on liver tissue protein oxidation and antioxidant enzyme levels after the exposure to radiofrequency radiation. Int J Radiat Biol (2015) 91(2):187–93.10.3109/09553002.2015.966210 [PubMed] [Cross Ref]176.
Li J, Meng Z, Zhang G, Xing Y, Feng L, Fan S, et al. N-acetylcysteine relieves oxidative stress and protects hippocampus of rat from radiation-induced apoptosis by inhibiting caspase-3. Biomed Pharmacother (2015) 70:1–6.10.1016/j.biopha.2014.12.029 [PubMed] [Cross Ref]177.
Kilciksiz S, Demirel C, Evirgen Ayhan S, Erdal N, Gurgul S, Tamer L, et al. N-acetylcysteine ameliorates nitrosative stress on radiation-inducible damage in rat liver. J BUON (2011) 16(1):154–9. [PubMed]178.
Demirel C, Kilciksiz S, Gurgul S, Erdal N, Yildiz A. N-acetylcysteine ameliorates gamma-radiation-induced deterioration of bone quality in the rat femur. J Int Med Res (2011) 39(6):2393–401.10.1177/147323001103900640 [PubMed] [Cross Ref]179.
Wang Y, Zhang ZZ, Chen SQ, Zou ZD, Tu XH, Wang L. [Protective effect of N-acetylcysteine on the intestinal barrier dysfunction after radiation injury in rats]. Zhonghua Wei Chang Wai Ke Za Zhi (2010) 13(3):219–22. [PubMed]180.
Demirel C, Kilciksiz S, Evirgen-Ayhan S, Gurgul S, Erdal N. The preventive effect of N-acetylcysteine on radiation-induced dermatitis in a rat model. J BUON (2010) 15(3):577–82. [PubMed]181.
Demirel C, Kilçiksiz S, Ay OI, Gürgül S, Ay ME, Erdal N. Effect of N-acetylcysteine on radiation-induced genotoxicity and cytotoxicity in rat bone marrow. J Radiat Res (2009) 50(1):43–50.10.1269/jrr.08066 [PubMed] [Cross Ref]182.
Wu W, Abraham L, Ogony J, Matthews R, Goldstein G, Ercal N. Effects of N-acetylcysteine amide (NACA), a thiol antioxidant on radiation-induced cytotoxicity in Chinese hamster ovary cells. Life Sci (2008) 82(21–22):1122–30.10.1016/j.lfs.2008.03.016 [PubMed] [Cross Ref]183.
Mansour HH, Hafez HF, Fahmy NM, Hanafi N. Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem Pharmacol (2008) 75(3):773–80.10.1016/j.bcp.2007.09.018 [PubMed] [Cross Ref]184.
Low WK, Sun L, Tan MG, Chua AW, Wang DY. l-N-acetylcysteine protects against radiation-induced apoptosis in a cochlear cell line. Acta Otolaryngol (2008) 128(4):440–5.10.1080/00016480701762490 [PubMed] [Cross Ref]185.
Kilciksiz S, Demirel C, Erdal N, Gürgül S, Tamer L, Ayaz L, et al. The effect of N-acetylcysteine on biomarkers for radiation-induced oxidative damage in a rat model. Acta Med Okayama (2008) 62(6):403–9. [PubMed]186.
Sminia P, van der Kracht AH, Frederiks WM, Jansen W. Hyperthermia, radiation carcinogenesis and the protective potential of vitamin A and N-acetylcysteine. J Cancer Res Clin Oncol (1996) 122(6):343–50.10.1007/BF01220801 [PubMed] [Cross Ref]187.
Tarbell NJ, Rosenblatt M, Amato DA, Hellman S. The effect of N-acetylcysteine inhalation on the tolerance to thoracic radiation in mice. Radiother Oncol (1986) 7(1):77–80.10.1016/S0167-8140(86)80126-8 [PubMed] [Cross Ref]188.
Kim JA, Baker DG, Hahn SS, Goodchild NT, Constable WC. Topical use of N-acetylcysteine for reduction of skin reaction to radiation therapy. Semin Oncol (1983) 10(1 Suppl 1):86–92. [PubMed]189.
Lieschke GJ, Ramenghi U, O’Connor MP, Sheridan W, Szer J, Morstyn G. Studies of oral neutrophil levels in patients receiving G-CSF after autologous marrow transplantation. Br J Haematol (1992) 82(3):589–95.10.1111/j.1365-2141.1992.tb06472.x [PubMed] [Cross Ref]190.
Dazzi C, Cariello A, Giovanis P, Monti M, Vertogen B, Leoni M, et al. Prophylaxis with GM-CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high-dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: a double blind, randomized, placebo-controlled study. Ann Oncol (2003) 14(4):559–63. [PubMed]191.
Cartee L, Petros WP, Rosner GL, Gilbert C, Moore S, Affronti ML, et al. Evaluation of GM-CSF mouthwash for prevention of chemotherapy-induced mucositis: a randomized, double-blind, dose-ranging study. Cytokine (1995) 7(5):471–7.10.1006/cyto.1995.0064 [PubMed] [Cross Ref]192.
McAleese JJ, Bishop KM, A’Hern R, Henk JM. Randomized phase II study of GM-CSF to reduce mucositis caused by accelerated radiotherapy of laryngeal cancer. Br J Radiol (2006) 79(943):608–13.10.1259/bjr/55190439 [PubMed] [Cross Ref]193.
Kannan V, Bapsy PP, Anantha N, Doval DC, Vaithianathan H, Banumathy G, et al. Efficacy and safety of granulocyte macrophage-colony stimulating factor (GM-CSF) on the frequency and severity of radiation mucositis in patients with head and neck carcinoma. Int J Radiat Oncol Biol Phys (1997) 37(5):1005–10.10.1016/S0360-3016(97)00105-3 [PubMed] [Cross Ref]194.
Sonis ST, Lindquist L, Van Vugt A, Stewart AA, Stam K, Qu GY, et al. Prevention of chemotherapy-induced ulcerative mucositis by transforming growth factor beta 3. Cancer Res (1994) 54(5):1135–8. [PubMed]195.
El-Habit OH, Saada HN, Azab KS, Abdel-Rahman M, El-Malah DF. The modifying effect of beta-carotene on gamma radiation-induced elevation of oxidative reactions and genotoxicity in male rats. Mutat Res (2000) 466(2):179–86.10.1016/S1383-5718(00)00010-3 [PubMed] [Cross Ref]196.
Konopacka M, Widel M, Rzeszowska-Wolny J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat Res (1998) 417(2–3):85–94.10.1016/S1383-5718(98)00095-3 [PubMed] [Cross Ref]197.
Mills EE. The modifying effect of beta-carotene on radiation and chemotherapy induced oral mucositis. Br J Cancer (1988) 57(4):416–7.10.1038/bjc.1988.94 [PMC free article] [PubMed] [Cross Ref]198.
Alfieri S, Ripamonti CI, Marceglia S, Orlandi E, Iacovelli NA, Granata R, et al. Temporal course and predictive factors of analgesic opioid requirement for chemoradiation-induced oral mucositis in oropharyngeal cancer. Head Neck (2016) 38(Suppl 1):E1521–7.10.1002/hed.24272 [PubMed] [Cross Ref]199.
Osaki T, Ueta E, Yoneda K, Hirota J, Yamamoto T. Prophylaxis of oral mucositis associated with chemoradiotherapy for oral carcinoma by azelastine hydrochloride (azelastine) with other antioxidants. Head Neck (1994) 16(4):331–9.10.1002/hed.2880160407 [PubMed] [Cross Ref]200.
Sato A, Saisho-Hattori T, Koizumi Y, Minegishi M, Iinuma K, Imaizumi M. Prophylaxis of mucosal toxicity by oral propantheline and cryotherapy in children with malignancies undergoing myeloablative chemo-radiotherapy. Tohoku J Exp Med (2006) 210(4):315–20.10.1620/tjem.210.315 [PubMed] [Cross Ref]201.
Mose S, Adamietz IA, Saran F, Thilmann C, Heyd R, Knecht R, et al. Can prophylactic application of immunoglobulin decrease radiotherapy-induced oral mucositis? Am J Clin Oncol (1997) 20(4):407–11.10.1097/00000421-199708000-00018 [PubMed] [Cross Ref]202.
Leborgne JH, Leborgne F, Zubizarreta E, Ortega B, Mezzera J. Corticosteroids and radiation mucositis in head and neck cancer. A double-blind placebo-controlled randomized trial. Radiother Oncol (1998) 47(2):145–8.10.1016/S0167-8140(97)00174-6 [PubMed] [Cross Ref]203.
Gruber S, Schmidt M, Bozsaky E, Wolfram K, Haagen J, Habelt B, et al. Modulation of radiation-induced oral mucositis by pentoxifylline: preclinical studies. Strahlenther Onkol (2015) 191(3):242–7.10.1007/s00066-014-0775-1 [PubMed] [Cross Ref]204.
Verdi CJ, Garewal HS, Koenig LM, Vaughn B, Burkhead T. A double-blind, randomized, placebo-controlled, crossover trial of pentoxifylline for the prevention of chemotherapy-induced oral mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (1995) 80(1):36–42.10.1016/S1079-2104(95)80014-X [PubMed] [Cross Ref]205.
Ferrà C, de Sanjosé S, Lastra CF, Martí F, Mariño EL, Sureda A, et al. Pentoxifylline, ciprofloxacin and prednisone failed to prevent transplant-related toxicities in bone marrow transplant recipients and were associated with an increased incidence of infectious complications. Bone Marrow Transplant (1997) 20(12):1075–80.10.1038/sj.bmt.1701023 [PubMed] [Cross Ref]206.
Lopez-Jimenez J, Cancelas JA, Valino JM, Garcia-Larana J, Garcia-Avello A, Arranz MI, et al. Effects of oral pentoxifylline on TNF-alpha levels, transplant-related toxicities and engraftment after bone marrow transplantation. Bone Marrow Transplant (1994) 14(6):1011–2. [PubMed]207.
Stockschläder M, Kalhs P, Peters S, Zeller W, Krüger W, Kabisch H, et al. Intravenous pentoxifylline failed to prevent transplant-related toxicities in allogeneic bone marrow transplant recipients. Bone Marrow Transplant (1993) 12(4):357–62. [PubMed]208.
Kalhs P, Lechner K, Stockschlader M, Kruger W, Peters S, Zander A. Pentoxifylline did not prevent transplant-related toxicity in 31 consecutive allogeneic bone marrow transplant recipients. Blood (1992) 80(10):2683–4. [PubMed]209.
Sathishkumar S, Boyanovsky B, Karakashian AA, Rozenova K, Giltiay NV, Kudrimoti M, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther (2005) 4(9):979–86.10.4161/cbt.4.9.1915 [PubMed] [Cross Ref]210.
Jaffrézou JP, Bruno AP, Moisand A, Levade T, Laurent G. Activation of a nuclear sphingomyelinase in radiation-induced apoptosis. FASEB J (2001) 15(1):123–33.10.1096/fj.00-0305com [PubMed] [Cross Ref]211.
Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res (2000) 60(2):321–7. [PubMed]212.
Miranda SR, Erlich S, Visser JW, Gatt S, Dagan A, Friedrich VL, Jr, et al. Bone marrow transplantation in acid sphingomyelinase-deficient mice: engraftment and cell migration into the brain as a function of radiation, age, and phenotype. Blood (1997) 90(1):444–52. [PubMed]213.
Chmura SJ, Mauceri HJ, Advani S, Heimann R, Beckett MA, Nodzenski E, et al. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor killing by ionizing radiation. Cancer Res (1997) 57(19):4340–7. [PubMed]214.
Santana P, Peña LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell (1996) 86(2):189–99.10.1016/S0092-8674(00)80091-4 [PubMed] [Cross Ref]215.
Levade T, Salvayre R, Potier M, Douste-Blazy L. Interindividual heterogeneity of molecular weight of human brain neutral sphingomyelinase determined by radiation inactivation method. Neurochem Res (1986) 11(8):1131–8.10.1007/BF00965942 [PubMed] [Cross Ref]216.
Levade T, Potier M, Salvayre R, Douste-Blazy L. Molecular weight of human brain neutral sphingomyelinase determined in situ by the radiation inactivation method. J Neurochem (1985) 45(2):630–2.10.1111/j.1471-4159.1985.tb04033.x [PubMed] [Cross Ref]217.
Cruz Éde P, Campos L, Pereira Fda S, Magliano GC, Benites BM, Arana-Chavez VE, et al. Clinical, biochemical and histological study of the effect of antimicrobial photodynamic therapy on oral mucositis induced by 5-fluorouracil in hamsters. Photodiagnosis Photodyn Ther (2015) 12(2):298–309.10.1016/j.pdpdt.2014.12.007 [PubMed] [Cross Ref]218.
Donnelly JP, Bellm LA, Epstein JB, Sonis ST, Symonds RP. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect Dis (2003) 3(7):405–12.10.1016/S1473-3099(03)00668-6 [PubMed] [Cross Ref]219.
Loury D, Embree JR, Steinberg DA, Sonis ST, Fiddes JC. Effect of local application of the antimicrobial peptide IB-367 on the incidence and severity of oral mucositis in hamsters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (1999) 87(5):544–51.10.1016/S1079-2104(99)70131-9 [PubMed] [Cross Ref]220.
Bondi E, Baroni C, Prete A, Gatti M, Carrassi A, Lodi G, et al. Local antimicrobial therapy of oral mucositis in paediatric patients undergoing bone marrow transplantation. Oral Oncol (1997) 33(5):322–6.10.1016/S1368-8375(97)00039-0 [PubMed] [Cross Ref]221.
Kuroda K, Caputo GA. Antimicrobial polymers as synthetic mimics of host-defense peptides. Wiley Interdiscip Rev Nanomed Nanobiotechnol (2013) 5(1):49–66.10.1002/wnan.1199 [PubMed] [Cross Ref]222.
Takahashi H, Palermo EF, Yasuhara K, Caputo GA, Kuroda K. Molecular design, structures, and activity of antimicrobial peptide-mimetic polymers. Macromol Biosci (2013) 13(10):1285–99.10.1002/mabi.201300126 [PMC free article] [PubMed] [Cross Ref]223.
Scott RW, Tew GN. Mimics of host defense proteins; strategies for translation to therapeutic applications. Curr Top Med Chem (2017) 17(5):576–89. [PubMed]224.
Nicolatou-Galitis O, Velegraki A, Sotiropoulou-Lontou A, Dardoufas K, Kouloulias V, Kyprianou K, et al. Effect of fluconazole antifungal prophylaxis on oral mucositis in head and neck cancer patients receiving radiotherapy. Support Care Cancer (2006) 14(1):44–51.10.1007/s00520-005-0835-2 [PubMed] [Cross Ref]225.
Lefebvre JL, Domenge C, Study Group of Mucositis . A comparative study of the efficacy and safety of fluconazole oral suspension and amphotericin B oral suspension in cancer patients with mucositis. Oral Oncol (2002) 38(4):337–42.10.1016/S1368-8375(01)00063-X [PubMed] [Cross Ref]226.
Shenep JL. Combination and single-agent empirical antibacterial therapy for febrile cancer patients with neutropenia and mucositis. NCI Monogr (1990) 9:117–22. [PubMed]227.
Spijkervet FK, Van Saene HK, Van Saene JJ, Panders AK, Vermey A, Mehta DM, et al. Effect of selective elimination of the oral flora on mucositis in irradiated head and neck cancer patients. J Surg Oncol (1991) 46(3):167–73.10.1002/jso.2930460309 [PubMed] [Cross Ref]228.
Matthews RH, Ercal N. Prevention of mucositis in irradiated head and neck cancer patients. J Exp Ther Oncol (1996) 1(2):135–8. [PubMed]229.
Spijkervet FK, van Saene HK, van Saene JJ, Panders AK, Vermey A, Mehta DM. Mucositis prevention by selective elimination of oral flora in irradiated head and neck cancer patients. J Oral Pathol Med (1990) 19(10):486–9.10.1111/j.1600-0714.1990.tb00792.x [PubMed] [Cross Ref]230.
Saral R, Burns WH, Prentice HG. Herpes virus infections: clinical manifestations and therapeutic strategies in immunocompromised patients. Clin Haematol (1984) 13(3):645–60. [PubMed]231.
Prelack MS, Patterson KR, Berger JR. Varicella zoster virus rhombencephalomyelitis following radiation therapy for oropharyngeal carcinoma. J Clin Neurosci (2016) 25:164–6.10.1016/j.jocn.2015.09.009 [PubMed] [Cross Ref]232.
Vaughan G, Rodríguez-Castillo A, Cruz-Rivera MY, Ruiz-Tovar K, Ramírez-González JE, Rivera-Osorio P, et al. Is ultra-violet radiation the main force shaping molecular evolution of varicella-zoster virus? Virol J (2011) 8:370.10.1186/1743-422X-8-370 [PMC free article] [PubMed] [Cross Ref]233.
Evans TG, Bernstein DI, Raborn GW, Harmenberg J, Kowalski J, Spruance SL. Double-blind, randomized, placebo-controlled study of topical 5% acyclovir-1% hydrocortisone cream (ME-609) for treatment of UV radiation-induced herpes labialis. Antimicrob Agents Chemother (2002) 46(6):1870–4.10.1128/AAC.46.6.1870-1874.2002 [PMC free article] [PubMed] [Cross Ref]234.
Jagetia GC, Aruna R, Nayak BS. Alteration in the radiation-induced LD release in HeLa cells by acyclovir. Clin Chim Acta (2000) 294(1–2):129–38.10.1016/S0009-8981(00)00180-7 [PubMed] [Cross Ref]235.
Jagetia GC, Aruna R. Effect of acyclovir on the radiation-induced micronuclei and cell death. Mutat Res (2000) 469(1):9–21.10.1016/S1383-5718(00)00048-6 [PubMed] [Cross Ref]236.
Bubley GJ, Chapman B, Chapman SK, Crumpacker CS, Schnipper LE. Effect of acyclovir on radiation- and chemotherapy-induced mouth lesions. Antimicrob Agents Chemother (1989) 33(6):862–5.10.1128/AAC.33.6.862 [PMC free article] [PubMed] [Cross Ref]237.
Spruance SL. Cutaneous herpes simplex virus lesions induced by ultraviolet radiation. A review of model systems and prophylactic therapy with oral acyclovir. Am J Med (1988) 85(2A):43–5. [PubMed]238.
Sougawa M, Akagi K, Murata T, Kawasaki S, Sawada S, Yoshii G, et al. Enhancement of radiation effects by acyclovir. Int J Radiat Oncol Biol Phys (1986) 12(8):1537–40.10.1016/0360-3016(86)90211-7 [PubMed] [Cross Ref]239.
Schmidt M, Haagen J, Noack R, Siegemund A, Gabriel P, Dörr W. Effects of bone marrow or mesenchymal stem cell transplantation on oral mucositis (mouse) induced by fractionated irradiation. Strahlenther Onkol (2014) 190(4):399–404.10.1007/s00066-013-0510-3 [PubMed] [Cross Ref]240.
Schmidt M, Piro-Hussong A, Siegemund A, Gabriel P, Dörr W. Modification of radiation-induced oral mucositis (mouse) by adult stem cell therapy: single-dose irradiation. Radiat Environ Biophys (2014) 53(4):629–34.10.1007/s00411-014-0552-7 [PubMed] [Cross Ref]241.
Shieh SH, Wang ST, Tsai ST, Tseng CC. Mouth care for nasopharyngeal cancer patients undergoing radiotherapy. Oral Oncol (1997) 33(1):36–41. [PubMed]242.
Rugg T, Saunders MI, Dische S. Smoking and mucosal reactions to radiotherapy. Br J Radiol (1990) 63(751):554–6.10.1259/0007-1285-63-751-554 [PubMed] [Cross Ref]243.
Scherlacher A, Beaufort-Spontin F. [Radiotherapy of head-neck neoplasms: prevention of inflammation of the mucosa by sucralfate treatment]. HNO (1990) 38(1):24–8. [PubMed]244.
Carter DL, Hebert ME, Smink K, Leopold KA, Clough RL, Brizel DM. Double blind randomized trial of sucralfate vs placebo during radical radiotherapy for head and neck cancers. Head Neck (1999) 21(8):760–6. [PubMed]245.
Feber T. Management of mucositis in oral irradiation. Clin Oncol (R Coll Radiol) (1996) 8(2):106–11. [PubMed]246.
Spijkervet FK, van Saene HK, Panders AK, Vermey A, van Saene JJ, Mehta DM, et al. Effect of chlorhexidine rinsing on the oropharyngeal ecology in patients with head and neck cancer who have irradiation mucositis. Oral Surg Oral Med Oral Pathol (1989) 67(2):154–61. [PubMed]247.
Ferretti GA, Raybould TP, Brown AT, Macdonald JS, Greenwood M, Maruyama Y, et al. Chlorhexidine prophylaxis for chemotherapy- and radiotherapy-induced stomatitis: a randomized double-blind trial. Oral Surg Oral Med Oral Pathol (1990) 69(3):331–8. [PubMed]248.
Hasenau C, Clasen BP, Roettger D. [Use of standardized oral hygiene in the prevention and therapy of mucositis in patients treated with radiochemotherapy of head and neck neoplasms]. Laryngol Rhinol Otol (Stuttg) (1988) 67(11):576–9. [PubMed]249.
Symonds RP, McIlroy P, Khorrami J, Paul J, Pyper E, Alcock SR, et al. The reduction of radiation mucositis by selective decontamination antibiotic pastilles: a placebo-controlled double-blind trial. Br J Cancer (1996) 74(2):312–7. [PMC free article] [PubMed]250.
Okuno SH, Foote RL, Loprinzi CL, Gulavita S, Sloan JA, Earle J, et al. A randomized trial of a nonabsorbable antibiotic lozenge given to alleviate radiation-induced mucositis. Cancer (1997) 79(11):2193–9. [PubMed]251.
Carl W, Emrich LS. Management of oral mucositis during local radiation and systemic chemotherapy: a study of 98 patients. J Prosthet Dent (1991) 66(3):361–9. [PubMed]252.
Abdelaal AS, Barker DS, Fergusson MM. Treatment for irradiation-induced mucositis. Lancet (1989) 1(8629):97. [PubMed]253.
Kim JH, Chu FC, Lakshmi V, Houde R. Benzydamine HCl, a new agent for the treatment of radiation mucositis of the oropharynx. Am J Clin Oncol (1986) 9(2):132–4. [PubMed]254.
Samaranayake LP, Robertson AG, MacFarlane TW, Hunter IP, MacFarlane G, Soutar DS, et al. The effect of chlorhexidine and benzydamine mouthwashes on mucositis induced by therapeutic irradiation. Clin Radiol (1988) 39(3):291–4. [PubMed]255.
Prada A, Chiesa F. Effects of benzydamine on the oral mucositis during antineoplastic radiotherapy and/or intra-arterial chemotherapy. Int J Tissue React (1987) 9(2):115–9. [PubMed]256.
Porteder H, Rausch E, Kment G, Watzek G, Matejka M, Sinzinger H. Local prostaglandin E2 in patients with oral malignancies undergoing chemo- and radiotherapy. J Craniomaxillofac Surg (1988) 16(8):371–4. [PubMed]257.
Maciejewski B, Zajusz A, Pilecki B, Swiatnicka J, Skladowski K, Dorr W, et al. Acute mucositis in the stimulated oral mucosa of patients during radiotherapy for head and neck cancer. Radiother Oncol (1991) 22(1):7–11. [PubMed]258.
Bourhis J, De Crevoisier R, Abdulkarim B, Deutsch E, Lusinchi A, Luboinski B, et al. A randomized study of very accelerated radiotherapy with and without amifostine in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys (2000) 46(5):1105–8. [PubMed]259.
Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C, Giatromanolaki A, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol (2000) 18(11):2226–33.10.1200/JCO.2000.18.11.2226 [PubMed] [Cross Ref]260.
Schonekas KG, Wagner W, Prott FJ. Amifostine—a radioprotector in locally advanced head and neck tumors. Strahlenther Onkol (1999) 175(Suppl 4):27–9. [PubMed]261.
Wagner W, Prott FJ, Schonekas KG. Amifostine: a radioprotector in locally advanced head and neck tumors. Oncol Rep (1998) 5(5):1255–7. [PubMed]262.
Buntzel J, Kuttner K, Frohlich D, Glatzel M. Selective cytoprotection with amifostine in concurrent radiochemotherapy for head and neck cancer. Ann Oncol (1998) 9(5):505–9. [PubMed]263.
Peters K, Mucke R, Hamann D, Ziegler PG, Fietkau R. Supportive use of amifostine in patients with head and neck tumors undergoing radio-chemotherapy. Is it possible to limit the duration of the application of amifostine? Strahlenther Onkol (1999) 175(Suppl 4):23–6. [PubMed]264.
Vacha P, Marx M, Engel A, Richter E, Feyerabend T. [Side effects of postoperative radiochemotherapy with amifostine versus radiochemotherapy alone in head and neck tumors. Preliminary results of a prospective randomized trial]. Strahlenther Onkol (1999) 175(Suppl 4):18–22. [PubMed]265.
Wagner W, Alfrink M, Haus U, Matt J. Treatment of irradiation-induced mucositis with growth factors (rhGM-CSF) in patients with head and neck cancer. Anticancer Res (1999) 19(1B):799–803. [PubMed]266.
Rosso M, Blasi G, Gherlone E, Rosso R. Effect of granulocyte-macrophage colony-stimulating factor on prevention of mucositis in head and neck cancer patients treated with chemo-radiotherapy. J Chemother (1997) 9(5):382–5.10.1179/joc.1997.9.5.382 [PubMed] [Cross Ref]267.
Mascarin M, Franchin G, Minatel E, Gobitti C, Talamini R, De Maria D, et al. The effect of granulocyte colony-stimulating factor on oral mucositis in head and neck cancer patients treated with hyperfractionated radiotherapy. Oral Oncol (1999) 35(2):203–8. [PubMed]268.
Schneider SB, Nishimura RD, Zimmerman RP, Tran L, Shiplacoff J, Tormey M, et al. Filgrastim (r-metHuG-CSF) and its potential use in the reduction of radiation-induced oropharyngeal mucositis: an interim look at a randomized, double-blind, placebo-controlled trial. Cytokines Cell Mol Ther (1999) 5(3):175–80. [PubMed]
Articles from Frontiers in Oncology are provided here courtesy of Frontiers Media SA
mucositis, mond- en keelkanker, radiotherapie, bestraling, levertraanzalf, levertraan, preventie, low dosis laser, aften
Gerelateerde artikelen
- FAQ - Veel gestelde vragen
- Sitehulp. Hoe vindt u informatie en artikelen op kanker-actueel?
- Wat is endometriose en hoe is dat te behandelen en kun je daarvan genezen?
- Waar in Europa wordt nog meer dendritische celtherapie gegeven? Hier een aantal adressen
- Wim Huppes staat 18 en 20 november 2024 terecht in de rechtbank Rotterdam nadat 4 nabestaanden en 1 overlevende hem hebben aangeklaagd
- Tineke geeft haar mening over het levenseinde in deze coronacrisis
- Crowdsurfing project: Helpen jullie mee met Keila en Yajaira en hun kinderen met het afbouwen van een winkeltje zodat zij in hun eigen levensonderhoud kunnen voorzien?
- Mevrouw B. en haar dochter met borstkanker en ook haar man hebben baat bij waterstofapparaat. Een ervaringsverslag
- Wat is een darmmicrobioomtest en wat zegt een uitslag over de status van je darmen en gezondheid in het algemeen?
- Wie heeft tips voor mij over hoe om te gaan met ziekte van Waldenstrom? Oncoloog wil zware chemo geven. Is er een alternatief?
- Een neuspray en mondspray zijn beschikbaar om te beschermen tegen het coronavirus - Covid-19. Te bestellen voor slechts 26 euro voor 1 maand gebruik per persoon.
- Wat kun je doen na het overleven van een levensbedreigende ziekte?
- 20 vragen die je aan de oncoloog of behandelend arts kunt stellen na een diagnose of een recidief van kanker. Samengesteld door Kees Braam
- Heeft er iemand ervaring met het innemen van petroleum tegen kanker?
- Is Chaga betula - betuline zuur interessant voor kankerpatienten?
- Vergroot deodorant de kans op borstkanker tijdens bestraling? Gerandomiseerde studies bewijzen van niet. Deodorant is veilig te gebruiken zegt ook arts-bioloog drs. Valstar.
- Wat is nu eigenlijk het verschil tussen adenocarcinomen, plaveiselcarcinomen, basaalcelcarcinomen, sarcomen en blastomen?
- Deelnemers gevraagd voor laagdrempelig onderzoek om zelf GC-Maf op te wekken door KEFIR plus BIEST (Bovine - Colostrum) te maken en gebruiken.
- Waar moet je op letten bij de aankoop van extracten van medicinale paddenstoelen? Hier wat richtlijnen
- Mijn ervaringen met dr. Robert Gorter en het Medisch Centrum Keulen. Een waarschuwing
- Is stopzetting van bepaalde complementaire behandelingen door CZ en andere ziektekostenverzekeraars terecht of een schaamteloze eenzijdige bezuiniging ten koste van patienten?
- Wat is nanothermia (= een vorm van hyperthermie met nanodeeltjes) en zijn er mensen die hier ervaring mee hebben?
- Zijn zoetstoffen schadelijk voor de gezondheid van de mens? Nieuw onderzoek ziet aantasting van darmflora door bepaalde zoetstoffen.
- Is Rode gist rijst (Red Yeast rice) beter dan statines om een hartinfarct te voorkomen? Arts-bioloog Engelbert Valstar analyseert aan de hand van de literatuur en kan Red Yeast Rice aanbevelen
- Waarom mag ik niet sterven als terminale kankerpatient?
- Laetrile - B-17 is dat een goed middel als preventie en bestrijding van kanker?
- Verzorging bij chemokuren. Tips van patienten hoe om te gaan met jezelf tijdens een chemokuur om deze beter te verdragen
- Maakt pijnstiller oxycodon van patienten een verslaafde? Is oxycodon de nieuwe nicotine? In Amerika stierven al honderduizenden mensen aan een overdosis.
- Hoe kun je mucositis - slijmvliesbeschadigingen in de mond door bestraling - radiotherapie voorkomen en behandelen?
- Immuuntherapie bij kanker. Hoe werkt dat nu precies?
- Wie heeft ervaring met intercostale neuralgie in relatie tot uitgezaaide borstkanker (bot-metastasen)? Weet iemand of hier koortsaanvallen bij horen en/of aantasting van de lever?
- Artsen zouden bijna allemaal chemo afwijzen als ze ongeneeslijk ziek zijn. Klopt die bewering wel?
- Welke man met teelbalkanker uit Limburg wil zijn verhaal vertellen aan journaliste van de Limburger?
- Diagnostische testen zoals bloedtesten, ontlastingstesten, urinetesten, weefseltesten enz, wat zijn dat? Waarvoor dienen ze? En kan ik die als patient ook zelf aanvragen?
- Hb of hemoglobine waarden: Mijn Hb is zo laag , hoe kan ik dat verbeteren?
- Wie heeft ervaring met verwijderen van de testikels om zo prostaatkanker te remmen?
- Wat is de meerwaarde van een 7-Tesla MRI in vergelijking met een gewone MRI?
- Waarom worden nog steeds hormonen als MPA en 17-B-Oestradiol toegestaan terwijl bekend is dat dit kanker veroorzaakt?
- Gelijke rechten door Jesaja de Vos: deel dit statement met wie je wilt
- Lijnzaadolie en hennepolie. Waarom hennepolie en lijnzaadolie goed zouden zijn?
- Neutropenie: Helpt neupogen of neulasta wel tegen neutropenie - tekort aan witte bloedlichaampjes - veroorzaakt door chemo? En zijn er niet toxische natuurlijke alternatieven voor?
- Dieetproblemen. Is het niet moeilijk om als kankerpatient het Houtsmullerdieet of Moermandieet te volgen en kun je dat van de ene op de andere dag zomaar doen?
- Wie heeft ervaring met dr. Rath? Ik heb longkanker
- Heeft iemand ervaring met gebruik van prostasol na hormoonresistentie van uitgezaaide prostaatkanker?
- Heeft iemand ervaring met crizotinib voor longkanker met ALK mutatie?
- Heeft iemand ervaring met papayabladeren naast chemo?
- Wie heeft ervaring met hypec behandeling voor eierstokkanker?
- Heeft iemand persoonlijk ervaring met NOVO TFF-100A voor hersentumoren?
- Ervaringen met alternatief niet altijd positief. Ook niet bij dr. Vogl. Een persoonlijke ervaring
- Levertraanzalf helpt goed tegen hand / voetsyndroom veroorzaakt door chemo, met name Xeloda - capecitabine
- De Mexicaanse griep en vaccineren. Arts-bioloog Engelbert Valstar trekt zijn conclusies op basis van wetenschappeliijke analyse en feiten
- Waarom hebben we de website kanker-actueel opgezet?
- Voedingssupplementen: een aantal vragen over bepaalde voedingsupplementen bij elkaar gezet
- Besteladressen: Waar kan ik voedingssupplementen bestellen? Hier enkele betrouwbare adressen, waar u ook korting kunt krijgen als u donateur wordt van kanker-actueel
- Orthomoleculaire arts. Heeft u het telefoonnummer van een betrouwbare orthomoleculaire arts?
- Houtsmullerdieet. Waarom zou je het Houtsmullerdieet gaan gebruiken als preventie van kanker?
- Vlees. Waarom is vlees slecht voor kankerpatiënten?
- Magnetron koken. Waarom is eten koken en opwarmen in de magnetron schadelijk?
- Sceptische arts. Mijn behandelend arts gelooft niet in diëten en vitamines. Hoe overtuig ik hem?
- Slechte eetgewoontes en verslavingen als roken en suiker.. Ik vind stoppen met roken zo moeilijk en hou zo van zoet!
- Hoe kom ik aan kopieën van artikelen en/of studierapporten die op kanker-actueel worden vermeld?
- Groene thee: Waarom is groene thee zo goed?
- Natuurarts - orthomoleculaire arts. Is een natuurarts hetzelfde als een orthomoleculaire arts?
- Antineoplastontherapie. Wat houdt een antineoplastontherapie in die dr. Burzynski gebruikt?
- Amastest. Wat is een AMAS test?
- Kahler - Multi Myeloma (MM). Waar kan ik info krijgen over Kahler - MM - Multi Myeloma?
- Hyperthermie. Wat is hyperthermie? En wat is het verschil tussen de verschillende vormen van hyperthermie?
- Waarom zijn omega vetzuren (bv. vette vis) goed voor een mens en vooral voor kankerpatiënten?
- Probiotica. Wat is probiotica en waar kan je het kopen?
- Wat zijn fase I, II en III studies en wat heb je er als deelnemende patient nog aan?
- Medicinale marihuana - cannabis. Hoe kom ik aan goeie marihuana of cannabis? Hier het antwoord.
- Tomaten en anti-oxidante werking door lycopeen. Waarom zijn tomaten gekookt beter dan ongekookt?
- Informatie over CT-scan en PET-scan op een rijtje gezet met o.a. de voordelen en nadelen van contrastvloeistof
- Wat kan ik doen tegen pijn veroorzaakt door mijn kanker? Zijn er ook niet toxische manieren van pijnbestrijding?
- Klachten in de gezondheidszorg. Waar kan ik terecht met mijn klachten over een slechte behandeling in een ziekenhuis en/of door artsen? Zie pagina nuttige adressen
- Sondevoeding zonder suiker. Kan ik ook sondevoeding krijgen zonder suiker die beter past in mijn dieet?






Plaats een reactie ...
Reageer op "Hoe kun je mucositis - slijmvliesbeschadigingen in de mond door bestraling - radiotherapie voorkomen en behandelen?"