Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
6 mei 2019: Lees ook dit artikel:
20 maart 2017: Bron: Het 2017 Multidisciplinary Thoracic Cancers Symposium in San Francisco, California
Uit een kleinschalige studie blijkt dat een eenvoudig uit te voeren bloedtest op in bloed circulerende tumorcellen (circulating tumor DNA (ctDNA)) gemiddeld een half jaar eerder een recidief of progressie van longkanker kan constateren in vergelijking met een CT-scan of Pet-scan. De onderzoekers benadrukken dat ondanks dat dit een kleinschalige studie was bij totaal 48 met succes behandelde longkankerpatiënten waarvan er 20 een recidief kregen, een biomarkertest voor verschil kan zorgen in het succesvol behandelen van een recidief van longkanker. Een bloedtest is namelijk eenvoudig uit te voeren en te combineren met CT-scan en/of Pet-scan die nu worden gebruikt om met succes behandelde longkankerpatiënbten te volgen, aldus de onderzoekers.
De studie werd afgelopen week gepresenteerd op het 2017 Multidisciplinary Thoracic Cancers Symposium in San Francisco.
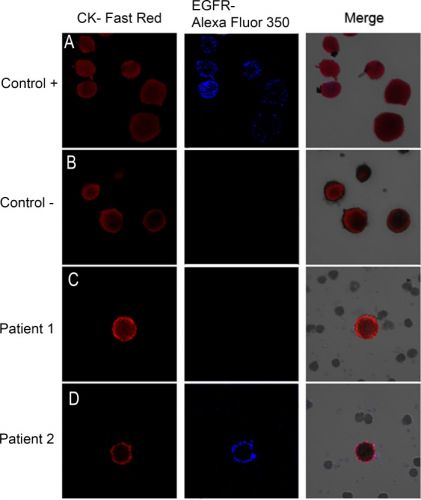
Hier een voorbeeld van enkele patiënten hoe zo'n bloedtest er op beeld uitziet. Tekst gaat verder onder beeld.
Image galleries after isolation, cytomorphological analysis, and detection of cytokeratin-positive (CK+) cells (red staining) and epidermal growth factor receptor (EGFR).
A) T1975 cell tumor line. was used as positive control for EGFR expression B) A control in which the slides are incubated with antibody diluent, without the primary antibody was included. (C, D) Expression of different markers in patients with non-small lung cancer through combination of stained CK+ cells (red) with EGFR (blue). EGFR-specific immunofluorescence (IF) of circulating tumor cells (CTCs) was determined with Alexa 355.
Kernpunten uit de studie waren:
- Van de 20 patienten die een recidief kregen hadden er 15 verhoogde in bloed circulerende tumorcellen. met een mediane tijd tot ziekteprogressie van 4,7 maanden in een range van 1,2 maanden tot een jaar.
- Van deze 15 patiënten demonstreerde 2/3 gemiddeld een half jaar eerder verhoogde in bloed circulerende tumorcellen in vergelijking met de CT-scan en/of Pet-scan een recidief aantoonde.
- Hoewel de meeste verhoogde tumorcellen in bloed eerder verscheen dan via beeldvorming bleken er ook 4 van de 20 patiënten een recidief te ontwikkelen, aangetoond met CT-scan en/of Pet-scan zonder dat er eerder stijgende tumorcellen in het bloed waren aangetoond.
Uit die laatste opmerking blijkt dan dat een biomarkertest toegevoegd zou moeten worden aan de nu gebruikte methode van CT-scan en Pet-scan. En volgens de onderzoekers is dit makkelijk en relatief goedkoop te realiseren.
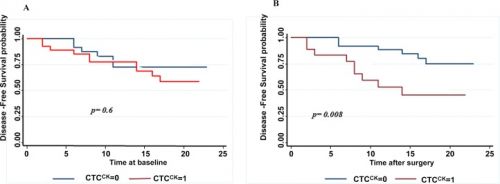
Een andere studie waarbij met succesvol geopereerde longkankerpatiënten werden gevolgd door een bloedtest en geeft min of meer dezelfde resultaten te zien maar dan op een andere manier. Als na een oparatie de in bloed circulerende tumorcellen drastisch daalde en niet verder steeg dan bleek de kans op een recidief vele malen kleiner dan wanneer de in bloed circulerende tumorcellen wel verhoogd bleken en weer na korte tijd begonnen te stijgen.Tekst gaat verder onder grafiek
Resultaten
51.8% of the patients evaluated were positive with the presence of CTCs at baseline. A decrease in the detection rate of CTCs was observed in these patients one month after surgery (32.1%) (p = 0.035). The mean number of CTCs was 3.16 per 10 ml (range 0–84) preoperatively and 0.66 (range 0–3) in postoperative determination. EGFR expression was found in 89.7% of the patients at baseline and in 38.9% patients one month after surgery. The presence of CTCs after surgery was significantly associated with early recurrence (p = 0.018) and a shorter disease free survival (DFS) (p = .008). In multivariate analysis CTC presence after surgery (HR = 5.750, 95% CI: 1.50–21.946, p = 0.010) and N status (HR = 0.296, 95% CI: 0.091–0.961, p = 0.043) were independent prognostic factors for DFS.
Het studierapport: Prospective Trial of Circulating Tumor Cells as a Biomarker for Early Detection of Recurrence in Patients with Locally Advanced None Small Cell Lung Cancer Treated with Chemoradiation Therapy staat als abstract no.3 in dit PDF rapport:
http://www.thoracicsymposium.org/uploadedFiles/Head_and_Neck_Symposium/Abstracts/FullAbstracts.pdf
Het volledige studierapport: Circulating Tumor Cells Identify Early Recurrence in Patients with Non-Small Cell Lung Cancer Undergoing Radical Resection is gratis in te zien. Het abstract staat hieronder.
CTCs can be detected and characterized in patients undergoing radical resection for non-small cell lung cancer. Their presence might be used to identify patients with increased risk of early recurrence.
Circulating Tumor Cells Identify Early Recurrence in Patients with Non-Small Cell Lung Cancer Undergoing Radical Resection
Abstract
Background
Surgery is the treatment of choice for patients with non-small cell lung cancer (NSCLC) stages I-IIIA. However, more than 20% of these patients develop recurrence and die due to their disease. The release of tumor cells into peripheral blood (CTCs) is one of the main causes of recurrence of cancer. The objectives of this study are to identify the prognostic value of the presence and characterization of CTCs in peripheral blood in patients undergoing radical resection for NSCLC.
Patients and Methods
56 patients who underwent radical surgery for previously untreated NSCLC were enrolled in this prospective study. Peripheral blood samples for CTC analysis were obtained before and one month after surgery. In addition CTCs were phenotypically characterized by epidermal growth factor receptor (EGFR) expression.
Results
51.8% of the patients evaluated were positive with the presence of CTCs at baseline. A decrease in the detection rate of CTCs was observed in these patients one month after surgery (32.1%) (p = 0.035). The mean number of CTCs was 3.16 per 10 ml (range 0–84) preoperatively and 0.66 (range 0–3) in postoperative determination. EGFR expression was found in 89.7% of the patients at baseline and in 38.9% patients one month after surgery. The presence of CTCs after surgery was significantly associated with early recurrence (p = 0.018) and a shorter disease free survival (DFS) (p = .008). In multivariate analysis CTC presence after surgery (HR = 5.750, 95% CI: 1.50–21.946, p = 0.010) and N status (HR = 0.296, 95% CI: 0.091–0.961, p = 0.043) were independent prognostic factors for DFS.
Conclusion
CTCs can be detected and characterized in patients undergoing radical resection for non-small cell lung cancer. Their presence might be used to identify patients with increased risk of early recurrence.
Discussion
The clinical value of CTCs detection in patients´ blood is becoming increasingly important as cancer biomarker research [14]. The main goal of CTC detection in early stages NSCLC is to identify patients with a high risk of recurrence after surgery in order to adopt the best strategy for treatment and follow up.
In this prospective study we found that CTC presence after surgery is associated with early recurrence and a shorter disease free survival in patients with surgical treatment of NSCLC
In our analysis, which enrolled 56 patients who had undergone radical surgery for previously untreated NSCLC, detection rate was significantly lower one month after surgery (32.14% vs. 51.79% before surgery, p = 0.034).
There are only a few studies that evaluate the prognostic significance of CTCs detection in resected NSCLC and even less that perform a second detection after surgery [10–13]. In a study by Hofman et al. [10] including I-IV stages resectable patients, the detection rate is similar to ours (49%). They perform only one presurgery CTCs detection employing a technique based on isolation by size (ISET technique), and they found that a CTC count of 50 cells or more is correlated with a worse overall and disease free survival. In another small scale study by Rolle et al. [11] 30 patients with resectable NSCLC were examined using the MAINTRAC technique, based on cytometric assay. CTCs were determined 2 weeks and 5 months after surgery. The CTCs count was compared to prognosis and patients with continuously increasing in median CTC-count post-operatively were shown to be at a higher risk of relapse.
Only two more prospective studies [12,13] using CellSearch system to detect CTCs, have reported so far the clinical impact of CTCs detection in peripheral blood before and after surgery. However, both failed to demonstrate any difference in either survival or recurrence. Both of them analyzed CTCs presence in blood immediately after surgery. Sawabata´s group [13] observed that CTCs disappeared ten days after operations. In fact, it is well known that during surgery a large number of CTCs can be shed into the blood stream but it has not been demonstrated that these cells are involved in the development of any future metastases. A number of them are apoptotic and many of them will be eliminated by the immune system [14]. That is why we think that for a real assessment of CTC presence after surgery the second detection must be performed at least 3 weeks after the operation.
Indeed we found a significant decrease in CTCs detection rate one month after surgery: CTCs were not detected in peripheral blood in almost 70% of patients in the second detection, and 25 out of 29 patients with CTCs presence at baseline showed a decrease in CTC count. This finding can be one of the reasons that explains why surgery remains the best treatment option in early stages.
The presence of CTCs one month after radical resection might indicate the presence of another source of CTCs not removed by surgery, such as those undetectable by conventional methods.
In accordance with that, in our study patients with detectable CTCs in peripheral blood one month after surgery presented a worse prognosis. Presence of CTCs after surgery (CTC2) was significantly associated with recurrence and DFS, so that CTC2 positive patients presented a higher risk of recurrence (p = 0.018) and a shorter DFS. The one year DFS rate was 51% in CTC2 positive patients compared to 87% for CTC2 negative patients (log rank test p = 0.008). CTC level was higher in patients with recurrence but the difference was not siginificant (mean number of 1 cell per 10 ml for patients with recurrence vs 0.5 per 10 ml for patients who did not develop a recurrence, p = 0.13).The mere presence of at least one CTCs after surgery (CTC2+) was significantly associated with patient prognosis in our analisys. Our results reflect that the presence of post-surgery CTCs (HR,5.75; 95% CI 1.50–21.95; p = 0.01) and nodal status (HR,3.38; 95% CI 1.04–10.9; p = 0.043) are good independent prognostic factors for DFS in multivariate analysis.
CTCs count in post-surgical samples showed an association with the max SUV of PET scan (p = .046). This is the first time that this association has been analysed. Max Suv value of PET scan has been associated with a worse prognoses and it could be related to a higher risk of postoperative CTCs presence.
Besides detection, CTCs characterization could be important to assess prognosis and the type of adjuvant treatment or to determine the best option of treatment for recurrent disease. EGFR expression was detected in 89.7% and 38.9% of CTCs isolated from blood samples taken before and after surgery, respectively. Regarding the prognostic siginificance of EGFR expression we found that 28.6% of patients with EGFR+CTC2+ experienced a recurrence compared to 63.6% for EGFR-CTC2+, but the difference was not significant. In our study CTCs detection after surgery was associated with worse prognosis but EGFR characterization was not. Probably a large-scale study may determine the EGFR expression influence in these patients prognosis.
On the other hand, it is worth stressing that current methodologies allow us to detect only the subpopulation of CTCs positive for epithelial markers, but not subpopulations positive for expression of mesenchymal markers and with a semi mesenchymal or mesenchymal phenotype. These phenotypes have been correlated with bad prognosis in different type of tumors [21] and therefore they should be analysed in future studies in order to achieve a better understanding of the prognostic role of CTCs in lung cancer.
To our knowledge, this study is the first large data set to validate CTCs as a prognostic marker performing postsurgery detection and to assess not only the risk of early recurrence but also to determine CTCs as predictive markers through their characterization.
In conclusion, our results show that CTCs can be detected in the blood of patients undergoing radical surgery for NSCLC. CTC detection after surgery could be an important prognostic marker contributing to risk stratification by identifying patients with a higher risk of early recurrence. Further studies are warranted to validate these results.
References
Gerelateerde artikelen
- Kunstmatige Intelligentie - Artificial intelligence kan op basis van samenvoeging van specifieke symptomen uit medisch dossier van de huisarts longkanker maanden eerder voorspellen
- Overlevingsmodel op basis van ML = machine learning voorspelt beter dan klinische stadiëring het risico op een recidief bij beginnende operabele longkanker stadium I tot III
- Longkanker: Circulerend tumor-DNA (ctDNA) regelmatig meten en combineren met een weefselanalyse (biopt) geeft beduidend meer informatie over veranderde mutaties bij patiënten met uitgezaaide gevorderde kleincellige longkanker copy 1
- Bronchoscopie met Cone beam CT-scan live 3D-beelden maakt betere diagnose van longkanker mogelijk en veel patiënten met verdachte afwijkingen in de longen kunnen profiteren van deze nieuwe methode
- Total body MRI gevolgd door verdere specifieke diagnose van uitgezaaide longkanker leidt sneller tot behandelplan dan standaard diagnose (13 vs 19 dagen) en was de helft goedkoper.
- CTC's : meting van in bloed circulerende tumorcellen vooraf aan behandeling voorspelt effectiviteit van chemo en bestraling bij patienten met niet-kleincellige longkanker.
- Eenvoudige bloedtest (biomarkertest) op in bloed circulerende tumorcellen ziet half jaar eerder recidief of progressie van succesvol behandelde longkanker dan CT-scan of Pet-scan
- DNA test, FISH methode gericht op ALK genen, zou gerichtere behandelingen van longkanker mogelijk kunnen maken.
- CT-scan geeft 33 procent vals positieve resultaten bij longkanker en lijkt daarmee onbetrouwbaar als meetinstrument voor longkanker.
- EUS-FNA - endoscopic ultrasound guided fine needle aspiration biopsy voorkomt onnodige longoperaties door veel nauwkeuriger diagnose
- Archief: Vroegtijdige screening van longkanker lijkt geen effect te hebben op langere overlevingsduur.
- Diagnose en oorzaak van longkanker: een overzicht van artikelen en recente ontwikkelingen. Scroll in linkerkolom voor artikelen



Plaats een reactie ...
Reageer op "Eenvoudige bloedtest (biomarkertest) op in bloed circulerende tumorcellen ziet half jaar eerder recidief of progressie van succesvol behandelde longkanker dan CT-scan of Pet-scan"