Zie ook in gerelateerde artikelen voor meer artikelen over immuuntherapie bij blaaskanker en urineleiderkanker
10 december 2021: Uit de vervolgstudie op de CHECKMATE274 fase III studie blijkt bij patiënten met hoog-risico spierinvasief urotheelcarcinoom (MIUC) (blaaskanker) die een volledige operatie hadden ondergaan en bij patiënten met een PD-L1-expressieniveau van ≥1%, aanvullende immuuntherapie met nivolumab de ziektevrije overleving te verbeteren. De mediane ziektevrije tijd in de intention-to-treat (ITT)-populatie was 20,8 maanden met nivolumab en 10,8 maanden met placebo. Deze gegevens veranderen de praktijk en leidden tot de goedkeuring door de Amerikaanse FDA van adjuvans nivolumab op 20 augustus 2021 bij patiënten met urotheelcarcinoom die een hoog risico lopen op een recidief na radicale resectie, ongeacht eerdere behandeling met neoadjuvante chemotherapie, betrokkenheid van de nodale of PD-L1-status.
Alle cijfers en details zijn te lezen in dit studieverslag gepubliceerd 2 december 2021, zie ook referenties onderaan dit artikel:
Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma

9 februari 2017: The Lancet 7 februari 20 17
Nivolumab, een anti-PD medicijn, gegeven aan patienten met gevorderde blaaskanker (urineleiderkanker) na resistentie van op platinum gebaseerde chemo zorgt alsnog voor uitstekende resultaten op progressievrije ziekte en verlenging van overall overleving. Zelfs bij patienten met weinig PD1 ligand expressie hadden voordeel van de nivolumab.
Nivolumab gaf een mediane remissie / respons te zien bij 19.6% van de patiënten (N = 270) met uitgezaaide blaaskanker na minstens 1 op platina gebaseerde chemokuur, inclusief 28.4%, 23.8%, en 16.1% respectievelijk bij degenen met een PD-L1 expressie van ≥ 5%, ≥ 1%, en < 1%, respectievelijk. De uiteindelijke resutlaten zullen nog beter worden want de mediane repons is nog niet bereikt op moment van analyse. Maar dat 1 op de 5 patienten alsnog een remissie bereikt is wel goed natuurlijk.
De FDA geeft op basis van deze studieresultaten uit de fase II CheckMate 275 studie ook officieel toestemming voor gebruik van nivolumab als immuuntherapie bij deze patiëntengroep. De studie werd gepubliceeerd in The Lancet Oncology
Voor het volledige studierapport: Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial moet betaald worden
Hieronder het abstract van de studie:
Nivolumab monotherapy provided meaningful clinical benefit, irrespective of PD-L1 expression, and was associated with an acceptable safety profile in previously treated patients with metastatic or surgically unresectable urothelial carcinoma.
Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial
Summary
Background
Patients with metastatic urothelial carcinoma have a dismal prognosis and few treatment options after first-line chemotherapy. Responses to second-line treatment are uncommon. We assessed nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor antibody, for safety and activity in patients with metastatic or surgically unresectable urothelial carcinoma whose disease progressed or recurred despite previous treatment with at least one platinum-based chemotherapy regimen.
Methods
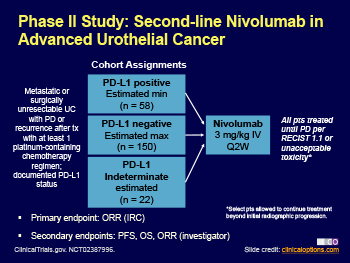
In this multicentre, phase 2, single-arm study, patients aged 18 years or older with metastatic or surgically unresectable locally advanced urothelial carcinoma, measurable disease (according to Response Evaluation Criteria In Solid Tumors v1.1), Eastern Cooperative Oncology Group performance statuses of 0 or 1, and available tumour samples for biomarker analysis received nivolumab 3 mg/kg intravenously every 2 weeks until disease progression and clinical deterioration, unacceptable toxicity, or other protocol-defined reasons. The primary endpoint was overall objective response confirmed by blinded independent review committee in all treated patients and by tumour PD-L1 expression (≥5% and ≥1%). This trial is registered with ClinicalTrials.gov, number NCT02387996, and is completed. Follow-up is still ongoing.
Findings
Between March 9, 2015, and Oct 16, 2015, 270 patients from 63 sites in 11 countries received nivolumab, and 265 were evaluated for activity. Median follow-up for overall survival was 7·00 months (IQR 2·96–8·77). Confirmed objective response was achieved in 52 (19·6%, 95% CI 15·0–24·9) of 265 patients. Confirmed objective response was achieved in 23 (28·4%, 95% CI 18·9–39·5) of the 81 patients with PD-L1 expression of 5% or greater, 29 (23·8%, 95% CI 16·5–32·3) of the 122 patients with PD-L1 expression of 1% or greater, and 23 (16·1%, 95% CI 10·5–23·1) of the 143 patients with PD-L1 expression of less than 1%. Grade 3–4 treatment-related adverse events occurred in 48 (18%) of 270 patients—most commonly grade 3 fatigue and diarrhoea, which each occurred in five patients. Three deaths were attributed to treatment (pneumonitis, acute respiratory failure, and cardiovascular failure).
Interpretation
Nivolumab monotherapy provided meaningful clinical benefit, irrespective of PD-L1 expression, and was associated with an acceptable safety profile in previously treated patients with metastatic or surgically unresectable urothelial carcinoma.
Funding
Bristol-Myers Squibb
References checkmate 274 study
REFERENCES
Gerelateerde artikelen
- Atezolizumab als eerstelijnstherapie voor patiënten met uitgezaaide of lokaal gevorderde urineleiderkanker geeft betere overall overleving maar niet statistisch significant in vergelijking met chemotherapie
- Atezolizumab, een anti-PD medicijn geeft uitstekende resultaten bij uitgezaaide en zwaar voorbehandelde patienten met urineleiderkanker en blaaskanker
- nivolumab plus gemcitabine en cisplatine verbetert overall overleving van patienten met blaaskanker blijkt uit de checkmate 901 studie
- Immuuntherapie met Tislelizumab gecombineerd met Nab-Paclitaxel voor niet-spierinvasief urnieleider - blaaskanker met hoog risico geeft bijzonder goede resultaten
- C-reactief proteïne bloedwaarden (CRP) heeft betere voorspellende waarde voor aanslaan van immuuntherapie dan PD-L1 expressie bij patiënten met uitgezaaide urineleiderkanker - blaaskanker
- Immuuntherapie met maandelijkse vaste dosis durvalumab geeft duurzame remissies bij eerder met chemotherapie behandelde patiënten met gevorderde blaaskanker - urineleiderkanker
- Immuuntherapie met nivolumab geeft uitstekende resultaten bij gevorderde uitgezaaide blaaskanker - urineleiderkanker
- Circulerend tumor-DNA voorspelt respons op immuuntherapie met anti-PD medicijn Atezolizumab bij spierinvasieve urineleiderkanker en blaaskanker
- Hoe eerder na chemo gestart met Avelumab, een immuuntherapeutisch medicijn als onderhoudsbehandeling,hoe beter de resultaten op ziektevrije tijd en overall overleving bij patienten met inoperabele blaaskanker en urineleiderkanker
- Immuuntherapie met gemoduleerd verkoudheidsvirus (Coxsackievirus A21) vooraf aan operatie van blaaskanker is succesvol
- Pembrolizumab - Keytruda - anti PD medicijn - geeft uitstekende resultaten bij gevorderde blaaskanker blijkt uit follow-up gegevens van eerder wegens succes stopgezette studie.
- Avelumab, een anti-PD medicijn door FDA goedgekeurd voor gebruik na falende chemo bij gevorderde urineleiderkanker - blaaskanker stadium 4
- Immuuntherapie met nivolumab en ipilimumab samen geeft hoopgevende resultaten bij zwaar voorbehandelde gevorderde uitgezaaide blaaskanker
- BCG - Bacillus Calmette-Guerin bij blaaskanker: Hier een mini overzicht gepubliceerd van wetenschappelijke studies en bewijzen over het gebruik van BCG - Bacillus Calmette-Gue´rin - als succesvolle immuuntherapie bij blaaskanker. Update 23 februari 2010
- Immuuntherapie bij blaaskanker: een overzicht inclusief immuuntherapie met anti-PD medicijnen - checkpointremmers





Plaats een reactie ...
Reageer op "Immuuntherapie met nivolumab geeft uitstekende resultaten bij gevorderde uitgezaaide blaaskanker - urineleiderkanker"