Update artikel 10 september 2017: Bron: ESMO 2017
Aanvullende resultaten gepresenteerd op ESMO 2017 d.d. 9 september 2017 van de eerder wegens uitstekende resultaten stopgezette studie bevestigen dat pembrolizumab ook op langerre termijn betere overall overleving en progressievrije ziekte geeft dan chemotherapie bij gevorderde blaaskanker.
Omdat de studie nog steeds de patiënten volgt is er nog geen statistische significantie bereikt maar die zal ongewtwijfeld wel bereikt worden omdat de patiënten die profiteren van pembrolizumab echt duurzame complete remissies en gedeeltelijke remissies bereiken. Onderaan dit artikel staat het abstract van de follow-up gegevens zoals die zijn gepresenteerd gisteren.
Dit zei dr. de Wit van het Erasmus Medisch Centrum, een van de hoofdonderzoekers over deze studie gisteren tijdens de presentatie:
The new data back up interim figures published earlier this year (2) and are “striking in the setting of urothelial cancer, which is highly lethal in the metastatic state,” said study investigator Dr Ronald de Wit, from Erasmus University Medical Center in Rotterdam, the Netherlands.
“Pembrolizumab is the first agent to improve survival over chemotherapy in the second-line setting. Not all patients benefit from checkpoint inhibition, but a sizeable proportion of patients who respond have very durable responses, even well over one year,” noted de Wit.
The phase 3 study randomly assigned patients whose urothelial cancer had recurred or progressed after platinum-based chemotherapy, to either pembrolizumab (n=272) or the investigator’s choice of paclitaxel, docetaxel, or vinflunine chemotherapy (n=270).
Results now out after 22.5 months of follow-up show an approximately three month advantage in overall survival (OS) in the pembrolizumab-treated patients compared to those receiving a second-line of chemotherapy (median 10.3 months vs. 7.4 months), with a further improvement in the hazard ratio from 0.73 to 0.70 (P = 0.0003) since the interim analysis, he said.
Median progression-free survival (PFS) was not significantly different (2.1 months for pembrolizumab vs 3.3; HR, 0.96; P = 0.32).
“Some patients also benefit from second line chemotherapy, but these responses tend to be short-lived and toxicity typically prevents prolonged treatment, whereas pembrolizumab is well tolerated,” de Wit said, adding that treatment-related adverse events of any grade occurred in 62.0% of pembrolizumab-treated patients compared to 90.6% of those treated with chemotherapy.
In addition, quality of life (QOL), measured at week 15 and reported earlier this year, showed better results in the pembrolizumab arm. “Overall, the superior survival, better adverse event profile, and better QOL render pembrolizumab a new standard of care in the second line treatment of urothelial cell cancer,” he concluded.
31 oktober 2016: Bron ESMO 2016
Pembrolizumab - Keytruda geeft uitstekende resultaten op progressievrije tijd en overall overleving bij gevorderde blaaskanker in vergelijking met of naast verschillende vormen van chemo.
“De resultaten van de KEYNOTE-045 studie betekenen een grote doorbraak en is belangrijk nieuws voor eerdere met chemotherapie behandelde patiënten met gevorderde blaaskanker.” zegt Roger M. Perlmutter, MD, PhD, President of Merck Research Laboratories. “We kijken ernaar uit om de studieresultaten te delen met de medische wereld en met de toezichthoudende autoriteiten over de hele wereld.”
Het veiligheidsprofiel van pembrolizumab in deze studie was consistent met dat gezien in eerdere studies met patiëntenj met gevorderde blaaskanker.

Resultaten
- Mediane leeftijd was 75 jaar (13% ≥85).
- 13% kreeg eerder pre-operatief een chemokuur.
- 87% had viscerale ziekte.
- 46% waren ECOG 2/3.
- 45% waren onbehandelbaar voor cisplatin wegens geen goede nierfunctie.
- 11% waren onbehandelbaar voor cisplatin wegens ECOG 2 performance status en geen goede nierfuncties.
- De CPS-high cutpoint (toelating op basis van receptorenexpressie) was vastgesteld op ≥10% PD-L1 expressie.
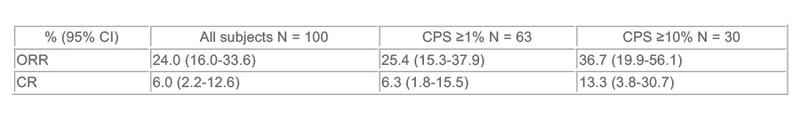
ORR - objective response was as follows:
Median duration of response (DOR) has not been reached (range, 1.4+ - 9.8+ mo). DOR rate ≥6 months was 83% (Kaplan-Meier estimate). 67% of pts experienced a drug-related adverse event (DRAE), most commonly fatigue (14%). 16% experienced a grade 3/4 DRAE. 5% discontinued therapy because of a DRAE.
Pembrolizumab - Keytruda is een zogeheten anti-PD medicijn, dat al goedgekeurd is voor o.a. borstkanker, longkanker , melanomen en hoofd- halstumoren zoals ook nivolumab en atezolizumab dat zijn.
De resultaten zijn dermate goed dat met instemming van de toezichthoudende autoriteiten de Keynote-045 studie wordt stopgezet omdat het ethisch niet verantwoord is de patiënten in de controlegroep en andere patiënten met blaaskanker deze klasse van medicijnen nog langer te onthouden.
Er is nog geen studierapport van verschenen maar zie hier het studieprotocol van de Keynote-045 studie en hieronder het abstract zoals dat op ESMO 2016 werd gepresenteerd.
Pembrolizumab was superior compared to investigator's choice of chemotherapy. And represent a major breakthrough and will be welcome news for patients dealing with previously treated advanced urothelial cancer
Source: ESMO 2016
Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: Preliminary results from the phase 2 KEYNOTE-052 study
A. Balar1, J. Bellmunt2, P.H. O'Donnell3, D. Castellano4, P. Grivas5, J. Vuky6, T. Powles7, E.R. Plimack8, N.M. Hahn9, R. de Wit10, L. Pang11, M.J. Savage12, R. Perini13, S. Keefe14, D. Bajorin15
1Medical Oncology, Perlmutter Cancer Center, NYU Langone Medical Center, New York, NY, USA, 2Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA, 3Dept. of Medicine, The University of Chicago Medical Centre, Chicago, IL, USA, 4Medical Oncology, Hospital Universitario 12 de Octubre, Madrid, Spain, 5Hematology/Oncology, Cleveland Clinic, Cleveland, OH, USA, 6Hematology and Oncology, Oregon Health Sciences University, Portland, OR, USA, 7Genitourinary Oncology, Barts Cancer Institute, Queen Mary University of London, London, UK, 8Department of Hematology/Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA, 9Oncology and Urology, Johns Hopkins University Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, USA, 10Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, Netherlands, 11BARDS Late Development Statistics, Merck & Co., Inc., Kenilworth, NJ, USA, 12Companion Diagnostics, Merck & Co., Inc., Kenilworth, NJ, USA, 13Medical Oncology, Merck & Co., Inc., Kenilworth, NJ, USA, 14Clinical Research & Development, Merck & Co., Inc., Kenilworth, NJ, USA, 15Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Background: KEYNOTE-052 (NCT02335424), an open-label, multicenter, phase 2 study, evaluated the efficacy and safety of pembro in first-line cisplatin-ineligible pts with u/m UC.
Methods: 374 pts have been enrolled. Eligibility included pathologically confirmed and measurable u/m UC, age ≥18 y, no chemotherapy for u/m disease, ECOG PS 0-2, and cisplatin ineligibility (ECOG PS 2, creatinine clearance <60 mL/min, ≥ grade 2 neuropathy or hearing loss, NYHA class III CHF). Pts received pembro 200 mg Q3W until progressive disease, unacceptable toxicity, or 24 mo of treatment. Primary end point was RECIST v1.1 confirmed objective response rate (ORR) by independent review in all pts and in PD-L1–positive pts by combined positive score (CPS) (tumor and immune cell PD-L1 expression). Secondary objective was to determine the CPS-high biomarker cutpoint. Interim analysis was planned to evaluate ORR for the first 100 pts and to determine the CPS-high cutpoint.
Results: Median age was 75 years (13% ≥85). 13% received perioperative chemotherapy. 87% had visceral disease. 46% were ECOG 2/3. 45% were cisplatin ineligible because of renal insufficiency only. 11% were cisplatin ineligible because of ECOG 2 performance status and renal insufficiency. The CPS-high cutpoint was determined to be ≥10% PD-L1 expression. As of 6/1/16, data cutoff (median 8 mo follow-up), ORR was as follows:
Median duration of response (DOR) has not been reached (range, 1.4+ - 9.8+ mo). DOR rate ≥6 months was 83% (Kaplan-Meier estimate). 67% of pts experienced a drug-related adverse event (DRAE), most commonly fatigue (14%). 16% experienced a grade 3/4 DRAE. 5% discontinued therapy because of a DRAE.
Conclusions: Pembro 200 mg Q3W demonstrates substantial antitumor activity and has a manageable toxicity profile in cisplatin-ineligible pts with u/m UC. CPS high cutpoint was determined to be ≥10% PD-L1 expression. CR rate of 6% for all pts and 13.3% for pts CPS ≥10% is encouraging.
Clinical trial identification: NCT02335424
Legal entity responsible for the study: Merck & Co. Inc.
Funding: Merck & Co., Inc.
Disclosure:
J. Bellmunt: Advisory board member for Merck, Genentech, Pfizer, Novartis, Sanofi, and Pierre Fabre, and research grants from Takeda, Novartis, and Sanofi.
P.H. O'Donnell: Advisory board member for Genentech, Astra Zeneca/Medimmune, and Merck, and research grants from Genentech, Merck, and Astra Zeneca/Medimmune.
P. Grivas: Advisory board member for Merck, Genentech, Bristol-Myers Squibb, Bayer, and Dendreon, and research grants from Merck, Genentech, Oncogenex, Bayer, Mirati, and Pfizer.
T. Powles: Honoraria from GlaxoSmithKline, Roche, Merck, and Bristol-Myers Squibb, and research grants from Roche/Genentech.
E.R. Plimack: Advisory board member for Acceleron, AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Eli Lilly Inc., Novartis, Pfizer, and Synergene, and research grants from Acceleron, AstraZeneca, Bristol-Myers Squibb, Eli Lilly Inc., Merck, and Pfizer.
N.M. Hahn: Advisory board member for AZ/MedImmune, Inovio, Pieris, Genentech/Roche, Merck, BMS, and OncoGeneX, and research grants to my institution from Novartis, BMS, Heat Biologics, Merck, Genentech/Roche, AZ/MedImmune, Principia Biopharma, Mirati, and OncoGeneX.
R. de Wit: Advisory board member for Sanofi, Merck, Lilly, and Roche, and research grants from Sanofi.
L. Pang, R. Perini: Employee of and own stock in Merck & Co., Inc.
M.J. Savage: Employee of and may own stock in Merck & Co., Inc.
S. Keefe: Employee of Merck & Co., Inc.
D. Bajorin: Advisory board member for Bristol-Myers Squibb, Roche, Merck, Genentech, Pfizer, and Novartis, and research grants from Roche, Merck, and Novartis.
All other authors have declared no conflicts of interest.
Mature Results Favour Pembrolizumab As Second-line Treatment For Bladder Cancer
Source: ESMO congress 2017
References
- Abstract LBA37_PR ‘Pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine for recurrent, advanced urothelial cancer (UC): mature results from the phase 3 KEYNOTE-045 trial’ will be presented by Dr Ronald de Wit during Poster Discussion Session ‘Genitourinary tumours, non-prostate’ on Sunday,10.09.2017, 14:45 - 16:15 (CEST), Cordoba Auditorium
- N Engl J Med 2017;376:1015-26
Disclaimer
This press release contains information provided by the authors of the highlighted abstracts and reflects the content of those abstracts. It does not necessarily reflect the views or opinions of ESMO who cannot be held responsible for the accuracy of the data. Commentators quoted in the press release are required to comply with the ESMO Declaration of Interests policy and the ESMO Code of Conduct .
About the European Society for Medical Oncology (ESMO)
ESMO is the leading professional organisation for medical oncology. With 16,000 members representing oncology professionals from over 130 countries worldwide, ESMO is the society of reference for oncology education and information. We are committed to supporting our members to develop and advance in a fast-evolving professional environment.
Abstract LBA37_PR
Pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine for recurrent, advanced urothelial cancer (UC): mature results from the phase 3 KEYNOTE-045 trial
R. de Wit1, D.J. Vaughn2, Y. Fradet3, J.-L. Lee4, L. Fong5, N.J. Vogelzang6, M.A. Climent7, D.P. Petrylak8, T.K. Choueiri9, A. Necchi10, W.R. Gerritsen11, H. Gurney12, D.I. Quinn13, S. Culine14, C.N. Sternberg15, Y. Mai16, M. Puhlmann16, R.F. Perini16, J. Bellmunt9, D.F. Bajorin17
1Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, Netherlands, 2Medical Oncology, Abramson Cancer Center of the University of Pennsylvania, Philadelphia, PA, USA, 3Medical Oncology, CHU de Québec-Université Laval, Québec City, QC, Canada, 4Medical Oncology, Asan Medical Center and University of Ulsan College of Medicine, Seoul, Korea, Republic of, 5Medical Oncology, University of California, San Francisco, San Francisco, CA, USA, 6Medical Oncology, Comprehensive Cancer Centers of Nevada, Las Vegas, NV, USA, 7Medical Oncology, Fundación Instituto Valenciano de Oncología, Valencia, Spain, 8Medical Oncology, Smilow Cancer Hospital at Yale University, New Haven, CT, USA, 9Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA, 10Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, 11Medical oncology, Radboud University Medical Center, Nijmegen, Netherlands, 12Medical Oncology, Westmead Hospital and Macquarie University, Sydney, NSW, Australia, 13Medical Oncology, University of Southern California Norris Comprehensive Cancer Center and Hospital, Los Angeles, CA, USA, 14Medical Oncology, Hôpital Saint-Louis, Paris, France, 15Medical Oncology, San Camillo Forlanini Hospital, Rome, Italy, 16Medical Oncology, Merck & Co., Inc., Kenilworth, NJ, USA, 17Medical Oncology, Memorial Sloan-Kettering Cancer Center, New York, USA
Background: In the phase 3 KEYNOTE-045 trial (NCT02256436), pembro was associated with significantly longer OS vs investigator’s choice of chemotherapy (chemo; paclitaxel, docetaxel, or vinflunine) in recurrent, advanced UC. Mature results from this open-label trial are presented.
Methods: Patients (pts) with histologically or cytologically confirmed UC, progression after platinum, ≤2 lines of systemic therapy, measurable disease (RECIST v1.1), and ECOG PS 0-2 were randomly assigned 1:1 to pembro 200 mg Q3W or paclitaxel 175 mg/m2 Q3W, docetaxel 75 mg/m2 Q3W, or vinflunine 320 mg/m2 Q3W. Primary end points: OS and PFS. Secondary end points included ORR and safety. Efficacy was assessed in all pts and pts with a PD-L1 combined positive score (CPS; % of PD-L1–expressing tumor and inflammatory cells) ≥10%.
Results: 270 pts assigned to pembro; 272 pts assigned to chemo. Baseline characteristics were generally similar between arms. As of May 19, 2017, median follow-up was 22.5 mo for both treatment arms. Median OS was significantly longer with pembro vs chemo in all pts (10.3 vs 7.4 mo; HR, 0.70; P = 0.0003) and in pts with CPS ≥10% (8.0 vs 5.2 mo; HR, 0.58; P = 0.003). OS was longer with pembro vs chemo regardless of age, liver metastases, hemoglobin, visceral disease, and choice of chemo. The 18-mo OS rate was 33.2% (95% CI, 27.5-38.9) with pembro vs 19.7% (95% CI, 14.7-24.8) with chemo (KM estimate). Median PFS was not significantly different (2.1 vs 3.3 mo; HR, 0.96; P = 0.32). ORR was 21.1% (95% CI, 16.4-26.5) (pembro) and 11.0% (95% CI, 7.6-15.4) (chemo). Responses were more durable with pembro (median response duration, not reached [1.6+ to 24.6+ mo] vs 4.4 mo [1.4+ to 24.0+]). Treatment-related AEs of any grade occurred in 62.0% (pembro) and 90.6% (chemo) of pts; grade ≥3 treatment-related AEs occurred in 16.5% and 50.2%.
Conclusions: With additional follow-up, OS with pembro vs chemo (paclitaxel, docetaxel, or vinflunine) continues to improve (HR, 0.70 vs 0.73 at Sept 7, 2016 data cutoff) and responses continue to be more durable with pembro. Pembro also continues to have a superior safety profile compared with chemo in pts with recurrent, advanced UC.
Clinical trial identification: ClinicalTrials.gov, NCT02256436, September 29, 2014
Legal entity responsible for the study: Merck & Co., Inc., Kenilworth, NJ, USA
Funding: Merck & Co., Inc., Kenilworth, NJ, USA
Disclosure: R. de Wit: Advisory board: Merck, Roche, Sanofi, Lilly
D.J. Vaughn: David Vaughn reports grants from Merck and personal fees from Astellas.
Y. Fradet: I have received research funding from Astellas and travel reimbursement from Roche and have served as consultant/advisor for Merck, Astellas, Roche, and AZ.
J.-L. Lee: Jae Lyun Lee reports personal fees from AstraZeneca, Astellas, Eisai and Pfizer.
L. Fong: Lawrence Fong reports grants from Merck, Dendreon, Bristol Myers Squibb, Roche-Genentech, Abbvie, and Amgen.
N.J. Vogelzang: Nicholas Vogelzang reports a consulting fee from Merck..
M.A. Climent: I have received honoraria from ROCHE, BMS, BAYER, ASTELLAS, SANOFI, JANSSEN, PFIZER, NOVARTIS , served as consultant/advisor for JANSSEN, PFIZER, ROCHE, SANOFI, ASTELLAS, BAYER, and travel reimbursement from ASTELLAS, JANSSEN, PFIZER
D.P. Petrylak: I have served as advisor for Bayer, Bellicum, Dendreon, Sanofi Aventis, Johnson and Johnson, Exelixis, Ferring, Millineum, Medivation, Pfizer, Roche Laboratories, (Tyme pharmaceuticals) and research funding from Oncogenix, Progenics, Johnson and Johnson, Merck, Millineum, Dendreon, Sanofi Aventis, Agensys, Eli Lilly, Roche Laboratories, and own stock in Bellicum and Tyme.
T.K. Choueiri: Toni K. Choueiri reports grants and personal fees from Merck and Pfizer.
A. Necchi: Andres Necchi reports grants and personal fees from Merck, Roche, Astra Zeneca, Bayer, Millennium Takeda, Amgen and Novartis.
W.R. Gerritsen: I have received research funding from Astellas, Bayer, and Janssen-Cilag and travel reimbursement from Amgen and Bayer, and have served as consultant/advisor for BMS, Amgen, MSD, Aglaia Biomedical Ventures, Astellas, Bayer, Janssen-Cilag
H. Gurney: I have received travel reimbursement from Astellas and served as consultant/advisor for BMS, GSK, Pfizer, and Astellas.
D.I. Quinn: David Quinn reports honoraria from Bayer, Astella, Novartis, Pfizer, Genentech/Roche, Merck, Merck Serono, Piramal, BMS, AstraZeneca, Dendreon, Exelixis, EMD Serono, and Sanofi and ad board acitivity from EMD Serono. He reports fees from a consulting or advisory role from Astellas Pharma, Novartis, Pfizer, BMS, Genentech/Roche, Merck Serono, Merck, Piramal, Bayer, Exelixis, AstraZeneca, Sanofi, Dendreon, and Peloton. Dr. Quinn also reports research funding from Millenium, Genentech/Roche, Sanofi, and GSK.
S. Culine: I have received research funding from Astellas, Roche, and MSD and travel reimbursement from Amgen, Astellas, and Janssen, and served as consultant/advisor for Roche and Janssen.
C.N. Sternberg: Cora Sternberg reports personal fees from Oncogenex along with grants and personal fees from Lilly, Janssen, Merck, BMS, Astra Zeneca, and Roche Genentech.
Y. Mai, M. Puhlmann: I am an employee of and own stock in Merck.
R.F. Perini: Rodolfo Perini reports being an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. In addition, Dr. Perini reports having a patent Application US 20160022814 A1 and 15101409 pending.
J. Bellmunt: I have received honoraria from Merck, Genentech, Pfizer, and AstraZeneca.
D.F. Bajorin: I have received research funding and travel reimbursement from Merck, Genentech, BMS, Roche, and Novartis, served as consultant/advisor for Merck, Genentech, Roche, Pfizer, AZ, and honoraria from Merck and Genentech
Gerelateerde artikelen
- Atezolizumab als eerstelijnstherapie voor patiënten met uitgezaaide of lokaal gevorderde urineleiderkanker geeft betere overall overleving maar niet statistisch significant in vergelijking met chemotherapie
- Atezolizumab, een anti-PD medicijn geeft uitstekende resultaten bij uitgezaaide en zwaar voorbehandelde patienten met urineleiderkanker en blaaskanker
- nivolumab plus gemcitabine en cisplatine verbetert overall overleving van patienten met blaaskanker blijkt uit de checkmate 901 studie
- Immuuntherapie met Tislelizumab gecombineerd met Nab-Paclitaxel voor niet-spierinvasief urnieleider - blaaskanker met hoog risico geeft bijzonder goede resultaten
- C-reactief proteïne bloedwaarden (CRP) heeft betere voorspellende waarde voor aanslaan van immuuntherapie dan PD-L1 expressie bij patiënten met uitgezaaide urineleiderkanker - blaaskanker
- Immuuntherapie met maandelijkse vaste dosis durvalumab geeft duurzame remissies bij eerder met chemotherapie behandelde patiënten met gevorderde blaaskanker - urineleiderkanker
- Immuuntherapie met nivolumab geeft uitstekende resultaten bij gevorderde uitgezaaide blaaskanker - urineleiderkanker
- Circulerend tumor-DNA voorspelt respons op immuuntherapie met anti-PD medicijn Atezolizumab bij spierinvasieve urineleiderkanker en blaaskanker
- Hoe eerder na chemo gestart met Avelumab, een immuuntherapeutisch medicijn als onderhoudsbehandeling,hoe beter de resultaten op ziektevrije tijd en overall overleving bij patienten met inoperabele blaaskanker en urineleiderkanker
- Immuuntherapie met gemoduleerd verkoudheidsvirus (Coxsackievirus A21) vooraf aan operatie van blaaskanker is succesvol
- Pembrolizumab - Keytruda - anti PD medicijn - geeft uitstekende resultaten bij gevorderde blaaskanker blijkt uit follow-up gegevens van eerder wegens succes stopgezette studie.
- Avelumab, een anti-PD medicijn door FDA goedgekeurd voor gebruik na falende chemo bij gevorderde urineleiderkanker - blaaskanker stadium 4
- Immuuntherapie met nivolumab en ipilimumab samen geeft hoopgevende resultaten bij zwaar voorbehandelde gevorderde uitgezaaide blaaskanker
- BCG - Bacillus Calmette-Guerin bij blaaskanker: Hier een mini overzicht gepubliceerd van wetenschappelijke studies en bewijzen over het gebruik van BCG - Bacillus Calmette-Gue´rin - als succesvolle immuuntherapie bij blaaskanker. Update 23 februari 2010
- Immuuntherapie bij blaaskanker: een overzicht inclusief immuuntherapie met anti-PD medicijnen - checkpointremmers



Plaats een reactie ...
Reageer op "Pembrolizumab - Keytruda - anti PD medicijn - geeft uitstekende resultaten bij gevorderde blaaskanker blijkt uit follow-up gegevens van eerder wegens succes stopgezette studie."