Helpt u ons aan 500 donateurs?
19 juni 2019: lees ook dit artikel:
Na het overlijden van Gerda najaar 2017 is de website opereren zonder snijden off line geweest en fucntioneerde niet meer. Samen met VUmc Amsterdam is de website volledig vernieuwd en patienten kunnen nu hun casus weer voorleggen aan een specialistisch team. Zie verder hieronder de informatie die nog steeds geldig is wat betreft aangeboden behandelingen enz.. Waarbij aangetekend dat sommige behandelingen nog zijn verbeterd of nieuwe aan toegevoegd.
16 april 2018: lees ook dit artikel:
25 maart 2018: Lees ook dit artikel eens:
Zie ook deze artikelen op kanker-actueel:
https://kanker-actueel.nl/NL/search.html?search_text=nanoknife&search_in=title
25 augustus 2015: Bron: Ann Surg. 2015 Sep;262(3):486-94. doi: 10.1097/SLA.0000000000001441.
Nanoknife - irreversable electroporation geeft bijzonder goede resultaten bij 200 patiënten met lokaal uitgezaaide alvleesklierkanker stadium III. Slechts 6% kreeg binnen de studieduur van 29 maanden een lokaal recidief. De mediane overall overleving blijkt nu al 24,9 maanden te bedragen (range: 4.9-85 maanden). De gemiddelde duur die patiënten in het ziekenhuis verbleven was 6 dagen. Bij 37% was er sprake van complicaties maar deze bleven grotendeels beperkt tot graad 2 en waren heel goed behandelbaar in het algemeen. Dit blijkt uit een Amerikaansew studie bij totaal 200 patiënten met lokaal uitgezaaide alvleesklierkanker stadium III. De nanoknife ingreep werd toegepast nadat alle patiënten eerst mediaan 6 maanden chemo en/of bestraling hadden gehad.
Studieresultaten:
Van juli 2010 tot oktober 2014, werden patiënten met radiografisch bewezen lokaal uitgezaaide alvleesklierkanker stadium III gevolgd in een over meerdere ziekenhuizen verspreide prospectieve studie.
Totaal 200 patiënten met lokaal uitgezaaide alvleesklierkanker werd of alleen met IRE - nanoknife behandeld (n = 150) of werden geopereerd waarin de IRE - nanonkife werd gebruikt om de snijvlakken schoon te krijgen (n = 50). Alle patiënten ondergingen vooraf chemotherapie en 52% van de patiënten kreeg er ook nog radiotherapie ernaast. Chemo en/of bestraling duurde mediaan 6 maanden (range, 5-13 maanden) voordat de IRE - nanoknife werd toegepast.
- IRE - nanoknife werd veilig en succesvol toegepast bij alle patiënten zonder belangrijke complicaties. 37% ervaarde wel complicaties maar deze bleven voor het grootste deel beperkt tot graad 2 (range 1 - 5) en waren in het algemeen goed behandelbaar.
- Mediaan verblijf in het ziekenhuis was 6 dagen (range, 4-36 dagen). Bij een mediane follow-up van 29 maanden, bleken 6 patiènten (3%) een lokaal recidief te hebben gekregen.
- Mediane overall overleving was na 29 maanden inmiddels 24.9 maanden (range: 4.9-85 maanden).
Conclusie:
Voor patiënten met lokaal uitgezaaide alvleesklierkanker (stadium III), resulteert de toevoeging van Irreversible electroporation aan chemo en/of bestraling pre operatief in een behoorlijke grote verbetering van de mediane overall overleving vergeleken met historische statistische gegevens. Deze resultaten suggereren dat een zogeheten ablatieve (niet invasieve operatie) van de primaire tumor overall overleving kan verbeteren.
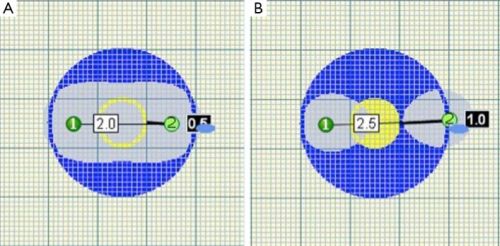
Foto: Plaatsing van de naalden bij IRE - nanoknife is heel belangrijk. er mag niet teveel tussenruimte zijn tussen de naalden.
Binnenkort komen ook resultaten van de eerste patiëntengroep van de PANFIRE studie die op ons initiatief samen met het SNFK is gestart. Maar er zijn afgelopen half jaar meerdere studies gepubliceerd over Irreversible electroporation - Nanoknife zoals deze: Use of irreversible electroporation in unresectable pancreatic cancer
of deze: Stage III pancreatic cancer and the role of irreversible electroporation
of deze: Systematic review of irreversible electroporation in the treatment of advanced pancreatic cancer.
En deze studie naar het effect van niet-vasieve ablatietechnieken uitgevoerd door o.a. dr. Martijn Meyerink en zijn collega's: Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer.
Abstracten van genoemde studies staan onderaan artikel.
Het volledige studierapport: Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients With Irreversible Electroporation: Safety and Efficacy. is tegen betaling in te zien.
Hier het abstract van deze studie:
For patients with LAPC - Locally Advanced Pancreatic Cancer (stage III), the addition of IRE to conventional chemotherapy and radiation therapy results in substantially prolonged survival compared with historical controls. These results suggest that ablative control of the primary tumor may prolong survival.
Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients With Irreversible Electroporation: Safety and Efficacy.
Abstract
OBJECTIVES:
Ablative therapies have been increasingly utilized in the treatment of locally advanced pancreatic cancer (LAPC). Irreversible electroporation (IRE) is an energy delivery system, effective in ablating tumors by inducing irreversible membrane destruction of cells. We aimed to demonstrate efficacy of treatment with IRE as part of multimodal treatment of LAPC.
METHODS:
From July 2010 to October 2014, patients with radiographic stage III LAPC were treated with IRE and monitored under a multicenter, prospective institutional review board-approved registry. Perioperative 90-day outcomes, local failure, and overall survival were recorded.
RESULTS:
A total of 200 patients with LAPC underwent IRE alone (n = 150) or pancreatic resection plus IRE for margin enhancement (n = 50). All patients underwent induction chemotherapy, and 52% received chemoradiation therapy as well for a median of 6 months (range, 5-13 months) before IRE. IRE was successfully performed in all patients. Thirty-seven percent of patients sustained complications, with a median grade of 2 (range, 1-5). Median length of stay was 6 days (range, 4-36 days). With a median follow-up of 29 months, 6 patients (3%) have experienced local recurrence. Median overall survival was 24.9 months (range: 4.9-85 months).
CONCLUSIONS:
For patients with LAPC (stage III), the addition of IRE to conventional chemotherapy and radiation therapy results in substantially prolonged survival compared with historical controls. These results suggest that ablative control of the primary tumor may prolong survival.
- PMID:
- 26258317
- [PubMed - in process]
Ablative therapies in patients with LAPC - locally advanced pancreatic cancer appear to be feasible and safe.
Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer.
Abstract
BACKGROUND:
Locally advanced pancreatic cancer (LAPC) is associated with a very poor prognosis. Current palliative (radio)chemotherapy provides only a marginal survival benefit of 2-3 months. Several innovative local ablative therapies have been explored as new treatment options. This systematic review aims to provide an overview of the clinical outcomes of these ablative therapies.
METHODS:
A systematic search in PubMed, Embase and the Cochrane Library was performed to identify clinical studies, published before 1 June 2014, involving ablative therapies in LAPC. Outcomes of interest were safety, survival, quality of life and pain.
RESULTS:
After screening 1037 articles, 38 clinical studies involving 1164 patients with LAPC, treated with ablative therapies, were included. These studies concerned radiofrequency ablation (RFA) (7 studies), irreversible electroporation (IRE) (4), stereotactic body radiation therapy (SBRT) (16), high-intensity focused ultrasound (HIFU) (5), iodine-125 (2), iodine-125-cryosurgery (2), photodynamic therapy (1) and microwave ablation (1). All strategies appeared to be feasible and safe. Outcomes for postoperative, procedure-related morbidity and mortality were reported only for RFA (4-22 and 0-11 per cent respectively), IRE (9-15 and 0-4 per cent) and SBRT (0-25 and 0 per cent). Median survival of up to 25·6, 20·2, 24·0 and 12·6 months was reported for RFA, IRE, SBRT and HIFU respectively. Pain relief was demonstrated for RFA, IRE, SBRT and HIFU. Quality-of-life outcomes were reported only for SBRT, and showed promising results.
CONCLUSION:
Ablative therapies in patients with LAPC appear to be feasible and safe.
© 2014 BJS Society Ltd. Published by John Wiley & Sons, Ltd.
Comment in
- PMID:
- 25524417
- [PubMed - indexed for MEDLINE]
Systematic review of novel ablative methods in locally advanced pancreatic cancer
Systematic review of novel ablative methods in locally advanced pancreatic cancer
Abstract
Unresectable locally advanced pancreatic cancer with or without metastatic disease is associated with a very poor prognosis. Current standard therapy is limited to chemotherapy or chemoradiotherapy. Few regimens have been shown to have a substantial survival advantage and novel treatment strategies are urgently needed. Thermal and laser based ablative techniques are widely used in many solid organ malignancies. Initial studies in the pancreas were associated with significant morbidity and mortality, which limited widespread adoption. Modifications to the various applications, in particular combining the techniques with high quality imaging such as computed tomography and intraoperative or endoscopic ultrasound has enabled real time treatment monitoring and significant improvements in safety. We conducted a systematic review of the literature up to October 2013. Initial studies suggest that ablative therapies may confer an additional survival benefit over best supportive care but randomised studies are required to validate these findings.
References
Gerelateerde artikelen
- Nanoknife - Irreversibele Elektroporatie Therapie (IRE) wordt ook bij prostaatkanker toegepast in St. Antonius Ziekenhuis copy 1
- Nanoknife - irreversible electroporation blijkt succesvolle operatietechniek te zijn bij lokaal uitgezaaide alvleesklierkanker. Slechts 6 patienten kreeg recidief op 2,5 jaar meting. copy 1
- IRE - irreversible electroporation - nanoknife blijkt veilige, nagenoeg niet-invasieve albatietechniek die bij inoperabele alvleesklierkanker en levertumoren voor uitstekende resultaten zorgt
- Studieprotocol voedingstudie als aanvulling op irreversible electroporation - nanoknife behandeling binnen de PANFIRE studie bij inoperabele alvleesklierkanker
- Martien vertelt over de succesvolle behandeling van zijn inoperabele alvleesklierkanker met irreversible electroporation - nanoknife techniek via de PANFIRE studie
- Vrouw met recidief van buikvlieskanker met inoperabele grote tumor toch succesvol geholpen met irreversible electrocorporation - nanoknife techniek
- Nanoknife - IRE - Irreversible electroporation blijkt succesvolle aanpak bij patiënten met inoperabele, onbehandelbare levertumoren.
- IRE - Irreversible electroporation in combinatie met chemo plus bestraling vooraf en achteraf bij patiënten met inoperabele vergevorderde alvleesklierkanker - stadium III geeft significante verbetering van ziektevrije tijd en overall overleving
- IRE - Irreversible electroporation, (nanoknife) een vorm van RFA maar dan met electropulsen, lijkt succesvolle en veilige operatie techniek te kunnen zijn bij niertumoren



Plaats een reactie ...
Reageer op "Nanoknife - irreversible electroporation blijkt succesvolle operatietechniek te zijn bij lokaal uitgezaaide alvleesklierkanker. Slechts 6 patienten kreeg recidief op 2,5 jaar meting. copy 1"