Helpt u ons aan 500 donateurs om kanker-actueel online te kunnen houden?
1 november 2018: Zie ook dit artikel:
1 augustus 2018: lees ook dit artikel:
4 april 2018: zie ook dit artikel:
6 november 2016:
Lees ook dit artikel:
30 september 2017: Lees ook dit artikel met de resultaten met de PARP remmer Niraparib bij eierstokkanker. En die resultaten waren ook echt goed te noemen, zelfs voor patiënten zonder BRCA mutatie.
30 september 2017: Bron: The Lancet
Olaparib geeft veel langere progressievrije overleving in vergelijking met een placebo bij patiënten met platinum chemo resistente eierstokkanker met een BRCA 1 / 2 mutatie blijkt uit tussenevaluatie van een nog steeds lopende studie die echter geen patienten meer aanneemt, maar wel de patiënten blijft volgen.
Vorig jaar meldde Astra Zenaca dit al op hun website, zie beschrijving hieronder d.d. 6 november 2016 en is inmiddels dus gepubliceerd in The Lancet in juli 2017.
Mediane proegressievrije tijd was 19·1 maanden [95% CI range 16·3 tot 25·7 maanden) versus 5·5 maanden [5·2 tot 5·8 maanden] voor de placebogroep; hazard ratio 0·30 [95% CI 0·22–0·41], p<0·0001)
Conclusie van de onderzoekers:
Een onderhoudsbehandeling met Olaparib tablet (in orale vorm) veroorzaakte een statistisch significante verbetering van de progressievrije overleving met geen schadelijke effecten op de kwaliteit van leven bij patiënten met platinum resistente eierstokkanker met een BRCA 1 / 2 mutatie. Behalve bij bloedarmoede (die vaker optrad bij olaparib 19 procent versus 2 procent)) waren de bijwerkingen gelijk in beide groepen en waren laaggradig en beheersbaar.
Het volledige studierapport: Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. is tegen betaling in te zien. Het abstract van de studie staat onderaan dit artikel:.
6 november 2016: Bron: AstraZeneca
Olaparib (Lynparza) verlengt progressievrije tijd en overall overall overleving beduidend beter in vergelijking met een placebo bij gevorderde eierstokkanker met de BRCA 1 en 2 mutaties. Althans dat meldt AstraZeneca op hun website en in een persbericht over de resultaten van de placebo gecontroleerde fase III studie genaamd SOLO-2.
De resultaten zouden in lijn zijn met eerdere resultaten uit studies met olaparib. Zie o.a. deze studie: Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy.
Of deze reviewstudie waarop de FDA olaparib officieel goedkeurde: FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy.
Echter van de SOLO-2 worden nog geen harde cijfers gegeven. Die worden op de eerstvolgende medische conferentie gegeven aldus AstraZeneca.
Ik kan dus nog geen cijfers geven maar hieronder het persbericht zoals dat ook op de website van AstraZeneca staat vermeld.
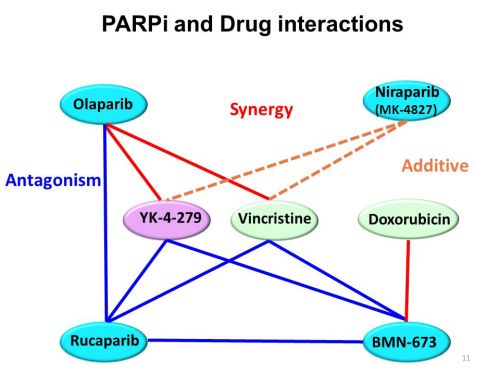
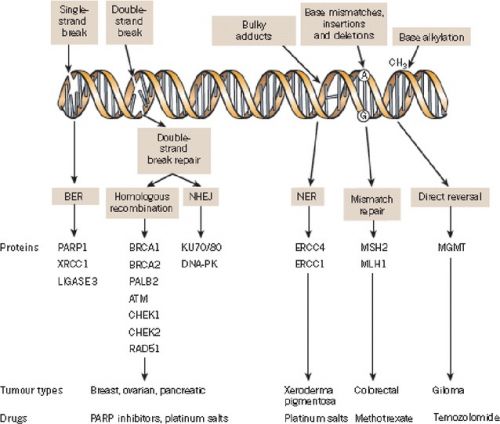
Interessant is ook deze studie die een overzicht geeft van de tot nu toe onderzochte PARP remmers en hun werkingsmechanisme met ook interessante referentielijst Update on Poly-ADP-ribose polymerase inhibition for ovarian cancer treatment
Verder is deze overzichtsstudie: Targeted therapy of ovarian cancer including immune check point inhibitor wellicht interessant eens te bekijken:.
In dit studierapport worden gerichte behandelingen (tageted therapies) zoals Avastin - Bevacizumab, Pazopanib, Cediranib en Parpremmers zoals olaparib en rucaparib of combinaties van beide soorten mediicijnen al of niet in combinatie met chemo besproken aan de hand van de literatuur - studies die tot nu toe daarmee zijn gedaan.
Ook immuuntherapie met anti-PD-medicijnen wordt in dit studierapport besproken. Zie onderaan dit artikel abstract en referentielijst van dit studierapport.
Hier het persbericht van Astrazenica over de SOLO-2 studie:
LYNPARZA™ (olaparib) Phase III SOLO-2 trial shows significant progression-free survival benefit
Source: AstrraZeneca
Trial studied LYNPARZA as maintenance treatment for women with BRCA-mutated metastatic ovarian cancer
Initial findings show safety profile with LYNPARZA tablets was consistent with previous studies
AstraZeneca today announced positive results from the Phase III SOLO-2 trial designed to determine the efficacy of LYNPARZATM (olaparib) tablets (300mg twice daily) as a monotherapy for the maintenance treatment of platinum-sensitive relapsed, BRCA-mutated ovarian cancer.1 Results from the trial demonstrate a clinically-meaningful and statistically-significant improvement of progression-free survival (PFS) among patients treated with LYNPARZA compared to placebo and provide additional evidence to support the potential use of LYNPARZA in this patient population.
Importantly, the median PFS in the LYNPARZA arm of SOLO-2 substantially exceeded that observed in the Phase II maintenance study in patients with platinum-sensitive relapsed ovarian cancer (Study 19).2
Sean Bohen, Executive Vice President, Global Medicines Development and Chief Medical Officer at AstraZeneca, said: “We are pleased with the robust improvement in progression-free survival demonstrated by LYNPARZA in the SOLO-2 trial. We will work with regulatory authorities to make LYNPARZA tablets available as quickly as possible to patients with ovarian cancer. We remain committed to investigating the full potential of LYNPARZA, both as monotherapy and in combinations, and to identifying all patients who may benefit from this important medicine.”
Initial findings demonstrate that safety profile with LYNPARZA tablets was consistent with previous studies.2 Full results of SOLO-2 will be presented at a forthcoming medical meeting.
Today’s positive results follow the Fast Track Designation for LYNPARZA by the US FDA earlier this year, in patients with a BRCA mutation who have platinum-sensitive, relapsed ovarian cancer.3
Olaparib tablet maintenance treatment provided a significant progression-free survival improvement with no detrimental effect on quality of life in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation. Apart from anaemia, toxicities with olaparib were low grade and manageable.
Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial.
Author information
- 1
- Medical Oncology Department, Université Paris Descartes, AP-HP, Paris, France. Electronic address: epujade@arcagy.org.
- 2
- Department of Oncology, University College London, London, UK.
- 3
- Hôpital Tenon, Paris, France.
- 4
- Department of Biostatistics and Research Methodology, University of Sydney, Sydney, NSW, Australia.
- 5
- Department of Hematology/Oncology, Massachusetts General Hospital, Boston, MA, USA.
- 6
- Princess Margaret Cancer Centre, Toronto, Canada.
- 7
- Gynecologic Oncology Department, Sheba Medical Center, Tel Aviv University, Tel Hashomer, Israel.
- 8
- Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland.
- 9
- Instituto Valenciano de Oncología, Valencia, Spain.
- 10
- Istituto Tumori Pascale di Napoli, Naples, Italy.
- 11
- Department of Medical Oncology, University of New South Wales Clinical School, Prince of Wales Hospital, Randwick, NSW, Australia.
- 12
- Gynecology Department, University of Milan-Bicocca and Istituto Europeo Oncología, Milan, Italy.
- 13
- Department of Gynecology and Gynecologic Oncology, Kliniken Essen Mitte, Essen, Germany.
- 14
- Department of Gynecologic Oncology, Saitama Medical University International Medical Center, Saitama, Japan.
- 15
- Medical Oncology Department, Centre Léon Bérard and University Claude Bernard, Lyon, France.
- 16
- The Royal Marsden NHS Foundation Trust, London, UK.
- 17
- Dana-Farber Cancer Institute, Boston, MA, USA.
- 18
- AstraZeneca, Gaithersburg, MD, USA.
- 19
- AstraZeneca, Cambridge, UK.
- 20
- Gustave Roussy Cancer Campus, Villejuif, France.
Abstract
BACKGROUND:
Olaparib, a poly(ADP-ribose) polymerase (PARP) inhibitor, has previously shown efficacy in a phase 2 study when given in capsule formulation to all-comer patients with platinum-sensitive, relapsed high-grade serous ovarian cancer. We aimed to confirm these findings in patients with a BRCA1 or BRCA2 (BRCA1/2) mutation using a tablet formulation of olaparib.
METHODS:
This international, multicentre, double-blind, randomised, placebo-controlled, phase 3 trial evaluated olaparib tablet maintenance treatment in platinum-sensitive, relapsed ovarian cancer patients with a BRCA1/2 mutation who had received at least two lines of previous chemotherapy. Eligible patients were aged 18 years or older with an Eastern Cooperative Oncology Group performance status at baseline of 0-1 and histologically confirmed, relapsed, high-grade serous ovarian cancer or high-grade endometrioid cancer, including primary peritoneal or fallopian tube cancer. Patients were randomly assigned 2:1 to olaparib (300 mg in two 150 mg tablets, twice daily) or matching placebo tablets using an interactive voice and web response system. Randomisation was stratified by response to previous platinum chemotherapy (complete vs partial) and length of platinum-free interval (6-12 months vs ≥12 months) and treatment assignment was masked for patients, those giving the interventions, data collectors, and data analysers. The primary endpoint was investigator-assessed progression-free survival and we report the primary analysis from this ongoing study. The efficacy analyses were done on the intention-to-treat population; safety analyses included patients who received at least one dose of study treatment. This trial is registered with ClinicalTrials.gov, number NCT01874353, and is ongoing and no longer recruiting patients.
FINDINGS:
Between Sept 3, 2013, and Nov 21, 2014, we enrolled 295 eligible patients who were randomly assigned to receive olaparib (n=196) or placebo (n=99). One patient in the olaparib group was randomised in error and did not receive study treatment. Investigator-assessed median progression-free survival was significantly longer with olaparib (19·1 months [95% CI 16·3-25·7]) than with placebo (5·5 months [5·2-5·8]; hazard ratio 0·30 [95% CI 0·22-0·41], p<0·0001). The most common adverse events of grade 3 or worse severity were anaemia (38 [19%] of 195 patients in the olaparib group vs two [2%] of 99 patients in the placebo group), fatigue or asthenia (eight [4%] vs two [2%]), and neutropenia (ten [5%] vs four [4%]). Serious adverse events were experienced by 35 (18%) patients in the olaparib group and eight (8%) patients in the placebo group. The most common in the olaparib group were anaemia (seven [4%] patients), abdominal pain (three [2%] patients), and intestinal obstruction (three [2%] patients). The most common in the placebo group were constipation (two [2%] patients) and intestinal obstruction (two [2%] patients). One (1%) patient in the olaparib group had a treatment-related adverse event (acute myeloid leukaemia) with an outcome of death.
INTERPRETATION:
Olaparib tablet maintenance treatment provided a significant progression-free survival improvement with no detrimental effect on quality of life in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation. Apart from anaemia, toxicities with olaparib were low grade and manageable.
FUNDING:
AstraZeneca.
Copyright © 2017 Elsevier Ltd. All rights reserved.
As demonstrated in recent clinical trials, the use of bevacizumab, cediranib, pazopanib, olaparib, and rucaparib, either alone or in combination with conventional cytotoxic agents, improves progression-free survival. Trials on immune checkpoint inhibitors such as nivolumab have revealed prolonged responses in a small set of ovarian cancer cases but require further exploration. In this review, we discuss the role of targeted therapies against ovarian cancer, including the use of immune checkpoint inhibitors.
Targeted therapy of ovarian cancer including immune check point inhibitor
Abstract
Epithelial ovarian cancer is the eighth most common cause of cancer-related deaths in women because most patients present with advanced stage disease at the time of diagnosis. Although cytoreductive surgery and platinum-based chemotherapy remain the gold standards of treatment, the recurrence rate of ovarian cancer remains high. Attempts to improve this standard two-drug chemotherapy by adding a third cytotoxic drug have failed to affect either progression-free survival or overall survival and have resulted in an increase in toxic side effects. Some anti-angiogenic agents, poly(ADP-ribose) polymerase, and immune checkpoint inhibitors have shown efficacy in early stages of development for the treatment of epithelial ovarian cancer. As demonstrated in recent clinical trials, the use of bevacizumab, cediranib, pazopanib, olaparib, and rucaparib, either alone or in combination with conventional cytotoxic agents, improves progression-free survival. Trials on immune checkpoint inhibitors such as nivolumab have revealed prolonged responses in a small set of ovarian cancer cases but require further exploration. In this review, we discuss the role of targeted therapies against ovarian cancer, including the use of immune checkpoint inhibitors
REFERENCES
Gerelateerde artikelen
- Parpremmers bij eierstokkanker: een meta analyse biedt een overzicht welke vorm van eierstokkanker gevoelig blijkt en welke niet. En hoe resistentie optreedt bespreekt een andere studie
- Parpremmers olaparib (Lynparza®) of niraparib (Zejula®) voor eierstokkanker en borstkanker worden gedeeltelijk uit basisverzekering gehaald door Zorginstituut Nederland copy 1
- Olaparib herhalen bij patienten met gevorderde eierstokkanker die al eerder olaparib en chemo hebben gehad blijkt alsnog effectief in vergelijking met placebo
- Niraparib als eerstelijns onderhoudsbehandeling bij patiënten met nieuw gediagnosticeerde gevorderde operabele eierstokkanker verdubbelt op 5 jaar ziektevrije overleving in vergelijking met placebo
- Niraparib - Zejula als onderhoudsbehandeling bij gevorderde eierstokkanker geeft een veel langere progressievrije overleving bij zowel patiënten met BRCA-gemuteerde eierstokkanker als bij niet BRCA-gemuteerd
- niraparib plus bevacizumab (Avastin) zonder chemo of gegeven in chemovrije periode verdubbelt bij eierstokkanker ziektevrije tijd en ziektevrije overleving steeg met 26 procent (79 vs 53 procent)
- Niraparib geeft zeer goede resultaten bij recidief van gevorderde eierstokkanker die eerder gevoelig bleek voor op platinum gebaseerde chemo copy 1
- Parpremmers Olaparib en niraparib gegeven als onderhoudsbehandeling bij gevorderde chemo gevoelige eierstokkanker heeft geen negatief effect op kwaliteit van leven en geeft wel betere overall overleving
- PARP remmers zoals olaparib zouden in vroeger stadium van eierstokkanker en andere vormen van kanker met BRCA mutaties moeten worden ingezet want teveel chemokuren verminderen kans op aanslaan van de behandeling, stellen oncologen n.a.v. diverse studies
- Talazoparib, een PARP remmer, wordt in veel studies bij veel verschillende vormen van kanker en in combinatie met andere medicijnen onderzocht en geeft veelbelovende resultaten copy 1
- Olaparib plus Bevacizumab als eerstelijns onderhoudsbehandeling voor eierstokkanker geeft betere progressievrije ziekte dan placebo ongeacht BRCA status.
- Olaparib als onderhoudsbehandeling voor BRCA 1/2 uitgezaaide platinum gevoelige eierstokkanker geeft 70 procent minder kans op overlijden in vergelijking met placebo
- Olaparib, een PARP remmer, verlengt ziektevrije en overall overleving in vergelijking met placebo bij eierstokkanker met BRCA 1 en 2
- Olaparib plus cedinarib lijkt doorbraak bij controle van vergevorderde eierstokkanker en verdubbelt progressievrije overleving van 9,2 maanden naar 17,7 maanden
- Parpremmer niraparib naast chemo en daarna als onderhoudsbehandeling verdubbelt mediane overall overleving in vergelijking met placebo
- Parpremmer Veliparib naast chemo gevolgd door veliparib alleen als onderhoudsbehandeling geeft betere overall overleving voor patienten met eierstokkanker stadium III en IV.
- Patienten met gevorderde eierstokkanker met een PARP-7 mutatie / expressie blijken veel betere mediane overall overleving te hebben (45 vs 16 maanden) dan zonder PARP-7 mutatie / expressie. copy 1
- Parpremmer rucaparib verdubbelt ziektevrije overleving (5 vs 11 en 13 maanden) bij chemo gevoelige eierstokkanker. Ook bij patienten zonder BRCA mutatie is rucaparib effectief
- PARP remmers zoals olaparib zouden in vroeger stadium van eierstokkanker en andere vormen van kanker met BRCA mutaties moeten worden ingezet
- BRCA - erfelijkheid: Combinatie van sapacitabine en seliciclib geeft een hoopvol therapeutisch effect bij zwaar voorbehandelde kankerpatienten met solide tumoren met onderliggende afwijkende erfelijke BCRA gen mutaties. copy 1
- PARP remmers zoals olaparib, niraparib en rucaparib zijn effectief zowel met als zonder BRCA mutaties, een overzicht



Plaats een reactie ...
Reageer op "Olaparib, een PARP remmer, verlengt ziektevrije en overall overleving in vergelijking met placebo bij eierstokkanker met BRCA 1 en 2"