Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
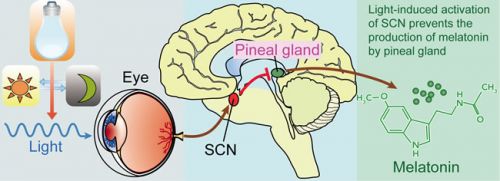
Foto: werkingsmechanisme van natuurlijke aanmaak van melatonine
16 juni 2016: Ik heb aan onderstaande meta analyse verschillende andere studies over effecten van melatonine toegevoegd. Het IGZ - Inspectie voor de Gezondheidszorg heeft ook informatie over melatonine maar nog geen update gemaakt van de beslissing van de Hoge Raad dat melatonine in dosering tot 5 mg. gewoon mag worden verkocht als voedingssupplement en niet hoeft te voldoen aan de eisen die aan een medicijn worden gesteld. Klik hier voor de uitspraak d.d. 1 juni 2016
Lees ook:
Voedingssupplementen met melatonine behouden voor consument
PERSBERICHT, Amersfoort, 2 juni 2016
Melatoninesupplementen mogen vanaf een bepaalde dosering niet zomaar als medicijn worden bestempeld. De Inspectie voor de Gezondheidszorg (IGZ) moet in de beoordeling of een product een geneesmiddel is, van geval tot geval, alle kenmerken van het product meenemen zoals de manier waarop het door de consument wordt gebruikt; om de gezondheid te bevorderen of om ziekte of een tekort op te heffen.
De Haagse rechter heeft deze uitspraak gisterenmiddag gedaan in de bodemprocedure die NPN heeft aangespannen tegen IGZ. Nederlanders gebruiken de lichaamseigen stof melatonine om jetlag te bestrijden of om beter in slaap te vallen. ”De uitspraak betekent een bescherming van het aanbod voedingssupplementen voor de consument.”, reageert Saskia Geurts, directeur van brancheorganisatie NPN. Lees verder>>>>>>>>>>
Onderstaande studies bewijzen dat melatonine wel degelijk werkzaam is als medicijn bij kanker. De abstracten en lange referentielijst (166 studies) staan onderaan dit artikel.
De volgende studie is uit 2015 en geeft gemiddeld genomen een voordeel van 28 procent versus 52 procent op 1-jaars overleving wanneer melatonine wordt gegeven naast chemo met ook een groot verschil in bijwerkingen. Let wel dit is een optelsom van een aantal gerandomiseerde studies die dus de overleving op 1 jaar bijna verdubbelen bij verschillende vormen van kanker. Hier een artikel over die studie: Melatonin could be an overlooked treatment for cancer
en als u klikt op de volgende PDF-link kunt u het studierapport lezen en of uitprinten: Melatonin and the influence on immune system and cancer
Een andere studie laat de meerwaarde zien van melatonine bij zowel chemo als bestraling ook dit is een reviewstudie: The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials.
En deze reviewstudie bij longkanker geeft uitstekende informatie over de werking van melatonine bij met name longkanker en longziektes in het studierapport: Melatonin as a potential anticarcinogen for non-small-cell lung cancer
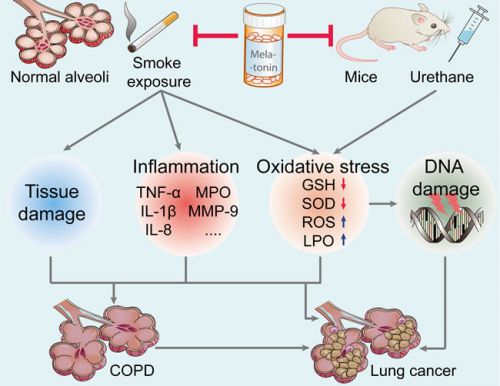
Zie hieronder een plaatje uit de studie bij longkanker en longziektes en hoe melatonine het schadelijke effecten van bv. roken herstelt of voorkomt:
Figure 2: Effect of melatonin on the prevention of lung carcinogenesis. Melatonin inhibits urethane-induced lung carcinogenesis in mice. Moreover, melatonin attenuates cigarette smoke-induced lung tissue damage, inflammation, and oxidative stress. Melatonin may reduce the incidence of lung cancer and lung diseases (such as COPD, a key risk factor for lung cancer). TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; MPO, myeloperoxidase; MMP-9, matrix metalloproteinase-9; GSH, glutathione; SOD, superoxide dismutase; ROS, reactive oxygen species; LPO, lipid peroxidation; COPD, chronic obstructive pulmonary diseases.
14 februari 2012: Bron: Cancer Chemother Pharmacol. 2012 Jan 24. [Epub ahead of print]
Een nieuwe meta analyse van tientallen gerandomiseerde studies bevestigt de meerwaarde van melatonine bij vele verschillende vormen van kanker, al of niet als aanvulling op chemo en bestraling - radiotherapie. Zo blijkt bv. dat de bijwerkingen van chemo en bestraling dramatisch minder zijn als daarbij 20 mg. melatonine per dag wordt gebruikt. Waaronder trombocytopenie (19,7 versus 2,2%; RR = 0,13, 95% CI, 0.06-0.28, P <0,00001), neurotoxiciteit (15,2 versus 2,5%; RR = 0,19, 95% CI, 0.09-0.40, P <0,0001) , en vermoeidheid (49,1 vs 17,2%; RR = 0,37, 95% CI, 0.28-0.48, P <0,00001). Maar ook de gedeeltelijke en volledige remissiecijfers verdubbelden (16,5 vs 32,6%; RR = 1,95, 95% CI, 1.49-2.54, P <0,00001) en ook de mediane 1-jaars overleving verbeterde significant (28,4 vs 52,2%; RR = 1,90; 95% CI, 1.28-2.83, P = 0,001). En deze meta analsye werd gedaan bij alle vormen van kanker dus niet 1 specifieke vorm. Klik hier voor adressen van goed gekwalificeerde orthomoleculaire artsen, die melatonine kunnen voorschrijven.
Hier het abstract van de studie. Als u hier klikt kunt u tegen betaling het volledige studirapport inzien.
Melatonin as an adjuvant therapy for cancer led to substantial improvements in tumor remission, 1-year survival, and alleviation of radiochemotherapy-related side effects.
The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials.
Source
Department of Pharmacy, The First Affiliated Hospital of Xinxiang Medical University, 88 Jiankang Road, The City of Weihui, Xinxiang, Henan Province, China.
Abstract
BACKGROUND:
Recently, melatonin has been associated with cancer both in vitro and in vivo. However, the value of melatonin in the treatment of cancer remains disputable. Hence, we performed a systematic review of randomized controlled trials (RCTs) of melatonin in solid tumor cancer patients and observed its effect on tumor remission, 1-year survival, and side effects due to radiochemotherapy.
METHODS:
An electronic search was conducted using the databases Pubmed, Medline, EMBASE, Cochrane library, and CNKI, from inception to November 2011. Trials using melatonin as adjunct treatment concurrent with chemotherapy or radiotherapy for cancer were included. Pooled relative risk (RR) for the tumor remission, 1-year survival, and radiochemotherapy-related side effects were calculated using the software Revman 5.0.
RESULTS:
The search strategy identified 8 eligible RCTs (n = 761), all of which studied solid tumor cancers. The dosage of melatonin used in the 8 included RCTs was 20 mg orally, once a day. Melatonin significantly improved the complete and partial remission (16.5 vs. 32.6%; RR = 1.95, 95% CI, 1.49-2.54; P < 0.00001) as well as 1-year survival rate (28.4 vs. 52.2%; RR = 1.90; 95% CI, 1.28-2.83; P = 0.001), and dramatically decreased radiochemotherapy-related side effects including thrombocytopenia (19.7 vs. 2.2%; RR = 0.13; 95% CI, 0.06-0.28; P < 0.00001), neurotoxicity (15.2 vs. 2.5%; RR = 0.19; 95% CI, 0.09-0.40; P < 0.0001), and fatigue (49.1 vs. 17.2%; RR = 0.37; 95% CI, 0.28-0.48; P < 0.00001). Effects were consistent across different types of cancer. No severe adverse events were reported.
CONCLUSIONS:
Melatonin as an adjuvant therapy for cancer led to substantial improvements in tumor remission, 1-year survival, and alleviation of radiochemotherapy-related side effects.
- PMID:
- 22271210
- [PubMed - as supplied by publisher]
Melatonin as a potential anticarcinogen for non-small-cell lung cancer
DOI: 10.18632/oncotarget.8776
Zhiqiang Ma1,*, Yang Yang2,3*, Chongxi Fan1,*, Jing Han4, Dongjin Wang2, Shouyin Di1, Wei Hu2, Dong Liu5, Xiaofei Li1, Russel J. Reiter6 and Xiaolong Yan1
1 Department of Thoracic Surgery, Tangdu Hospital, The Fourth Military Medical University, Xi’an, China
2 Department of Thoracic and Cardiovascular Surgery, Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China
3 Department of Biomedical Engineering, The Fourth Military Medical University, Xi’an, China
4 Department of Ophthalmology, Tangdu Hospital, The Fourth Military Medical University, Xi’an, China
5 State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
6 Department of Cellular and Structural Biology, UT Health Science Center, San Antonio, TX, USA
* These authors have contributed equally to this work.
Correspondence to:
Xiaolong Yan, email: xiaolongyanfmmu@126.com
Russel J. Reiter, email: reiter@uthscsa.edu
Xiaofei Li, email: xiaofeilitangdu@126.com
Keywords: melatonin; non-small-cell lung cancer; oncostatic effects; drug synergy; potential directions
Received: December 08, 2015 Accepted: March 31, 2016 Published: April 18, 2016
Table 2:The drug synergy of melatonin in NSCLC
|
Cancer categories |
Number of patients |
Drugs and dose |
Outcome |
References |
|
Experimental studies |
||||
|
SK-LU-1 NSCLC cell line |
None |
Melatonin (1, 2 mM) + cisplatin (10-200 μM) (48 h in culture) |
In the drug combination, 1 and 2 mM melatonin reduced IC50 concentration of cisplatin alone from 50 μM to 11 and 4 μM. Thus, melatonin enhances cisplatin-induced cytotoxicity and apoptosis in SK-LU-1 cells and induces cell cycle arrest in the S phase in contrast to cisplatin alone group |
Plaimee et al. [5] |
|
A549 cells and healthy human lymphocytes |
None |
Melatonin (50 μM) + irinotecan (7.5, 15, 30, and 60 μM) |
The combination treatment resulted in an increase in the amount of DNA damage in A549 cells, but was not effective in inducing DNA damage in healthy human lymphocytes |
Kontek et al. [122] |
|
A549 cells |
None |
Melatonin (0.1, 1 mM) + doxorubicin (0.1, 1 microg/ml) |
Melatonin intensified cytotoxicity of doxorubicin in all cell lines, significantly decreasing cell numbers and promoting apoptosis |
Fic et al. [124] |
|
Female C57B/6 mice with subcutaneous propagation of Lewis lung carcinoma |
None |
Melatonin (1 mg/kg) + cyclophosphamide (40, 160 mg/kg) + etoposide (20, 40 mg/kg) |
Melatonin can rescue myeloid progenitor cells from chemotherapy-induced apoptosis via a mechanism involving the endogenous production of GM-CSF by T cells |
Maestroni et al. [114] |
|
H1299 and A549 cells |
None |
Melatonin (1 mM) + berberine (20μM to 200 μM) |
Melatonin sensitized NSCLC cells to berberine and enhanced the growth inhibitory effect of berberine by activating caspase/Cyto C and inhibiting AP-2β/hTERT, NF-κB/COX-2 and Akt/ERK signaling pathways |
Lu et al. [125] |
|
Clinical trials |
||||
|
Untreatable metastatic NSCLC or GI cancers |
846 |
Melatonin (20 mg/day) + IL-2 (3 million IU/day, 5 days/week, 4 weeks) + supportive care |
The combination treatment provided a further improvement in the percentage of tumor regressions and of 3-year survival with respect to melatonin or supportive care alone |
Lissoni et al. [127] |
|
Advanced lung adenocarcinoma |
23 |
Melatonin (20 mg/day) + somatostatin (1-3 mg/day) + Retinoids (5 ml) + Vitamin D (0.3 mg/day) + bromocriptine (2.5 mg/day) + cyclophosphamide (150 mg/day) |
Patients with combination treatment had a median overall survival of 95 days, with very modest toxic effects and an improvement in both respiratory and general symptoms associated with length of survival |
Norsa et al. [123] |
|
Untreated metastatic NSCLC |
147 |
Melatonin (20 mg/day) + cisplatin plus etoposide or gemcitabine |
The 2-year survival rate and the overall tumor regression rate achieved in patients concomitantly treated with melatonin was significantly higher than that found in those treated with chemotherapy alone |
Lissoni et al. [33] |
|
Untreated metastatic NSCLC |
100 |
Melatonin (20 mg/day) + cisplatin (20 mg/m2/day) + etoposide (100 mg/m2/day) |
Overall tumor regression rate and the 5-year survival results (49%) were significantly higher in patients concomitantly treated with melatonin. In particular, no patient treated with chemotherapy alone was alive after 2 years |
Lissoni et al. [8] |
|
Advanced NSCLC |
70 |
Melatonin (20 mg/day) + cisplatin (20 mg/m2/day) + etoposide (100 mg/m2/day) |
The percent of 1-year survival was significantly higher in patients treated with melatonin plus chemotherapy than in those who received chemotherapy alone (15/34 vs. 7/36, P <0.05) |
Lissoni et al. [129] |
GM-CSF, granulocyte-macrophage colony-stimulating factor; Cyto C, cytochrome C; AP-2β, activator protein 2β; hTERT, telomerase reverses transcriptase; NF-κB, nuclear factor κB; COX-2, cyclooxygenase 2; ERK, extracellular signal-regulated kinase
Abstract
Non-small-cell lung cancer (NSCLC) is a leading cause of death from cancer worldwide. Melatonin, an idoleamine discovered in the pineal gland, exerts pleiotropic anticancer effects against a variety of cancer types. In particular, melatonin may be an important anticancer drug in the treatment of NSCLC. Herein, we review the correlation between the disruption of the melatonin rhythm and NSCLC incidence; we also evaluate the evidence related to the effects of melatonin in inhibiting lung carcinogenesis. Special focus is placed on the oncostatic effects of melatonin, including anti-proliferation, induction of apoptosis, inhibition of invasion and metastasis, and enhancement of immunomodulation. We suggest the drug synergy of melatonin with radio- or chemotherapy for NSCLC could prove to be useful. Taken together, the information complied herein may serve as a comprehensive reference for the anticancer mechanisms of melatonin against NSCLC, and may be helpful for the design of future experimental research and for advancing melatonin as a therapeutic agent for NSCLC.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87-108.
2. Bender E. Epidemiology: The dominant malignancy. Nature. 2014; 513:S2-3.
3. Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Govindan R, Grannis FW, Jr., Grant SC, Horn L, Jahan TM, Komaki R, Kong FM, Kris MG, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013; 11:645-653; quiz 653.
4. Johnson DH, Schiller JH, Bunn PA, Jr. Recent clinical advances in lung cancer management. J Clin Oncol. 2014; 32:973-982.
5. Plaimee P, Weerapreeyakul N, Barusrux S, Johns NP. Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells. Cell Prolif. 2015; 48:67-77.
6. Kim W, Jeong JW, Kim JE. CCAR2 deficiency augments genotoxic stress-induced apoptosis in the presence of melatonin in non-small cell lung cancer cells. Tumour Biol. 2014; 35:10919-10929.
7. Sener G, Jahovic N, Tosun O, Atasoy BM, Yegen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003; 74:563-572.
8. Lissoni P, Chilelli M, Villa S, Cerizza L, Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003; 35:12-15.
9. Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010; 181:127-151.
10. Gobbo MG, Dizeyi N, Abrahamsson PA, Bertilsson PA, Masiteli VS, Pytlowanciv EZ, Taboga SR, Goes RM. Influence of Melatonin on the Proliferative and Apoptotic Responses of the Prostate under Normal and Hyperglycemic Conditions. J Diabetes Res. 2015; 2015:538529.
11. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules. 2015; 20:18886-18906.
12. Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001; 34:237-256.
13. Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 2015; 59:403-419.
14. Fan C, Pan Y, Yang Y, Di S, Jiang S, Ma Z, Li T, Zhang Z, Li W, Li X, Reiter RJ, Yan X. HDAC1 inhibition by melatonin leads to suppression of lung adenocarcinoma cells via induction of oxidative stress and activation of apoptotic pathways. J Pineal Res. 2015; 59:321-333.
15. Plaimee P, Weerapreeyakul N, Thumanu K, Tanthanuch W, Barusrux S. Melatonin induces apoptosis through biomolecular changes, in SK-LU-1 human lung adenocarcinoma cells. Cell Prolif. 2014; 47:564-577.
16. Alvarez-Garcia V, Gonzalez A, Alonso-Gonzalez C, Martinez-Campa C, Cos S. Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J Pineal Res. 2013; 54:373-380.
17. Borin TF, Arbab AS, Gelaleti GB, Ferreira LC, Moschetta MG, Jardim-Perassi BV, Iskander A, Varma NR, Shankar A, Coimbra VB, Fabri VA, de Oliveira JG, Zuccari DA. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J Pineal Res. 2016; 60:3-15.
18. Woo SM, Min KJ, Kwon TK. Melatonin-mediated Bim up-regulation and cyclooxygenase-2 (COX-2) down-regulation enhances tunicamycin-induced apoptosis in MDA-MB-231 cells. J Pineal Res. 2015; 58:310-320.
19. Alonso-Gonzalez C, Gonzalez A, Martinez-Campa C, Gomez-Arozamena J, Cos S. Melatonin sensitizes human breast cancer cells to ionizing radiation by downregulating proteins involved in double-strand DNA break repair. J Pineal Res. 2015; 58:189-197.
20. Proietti S, Cucina A, Dobrowolny G, D’Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A, Morini V, Reiter RJ, Bizzarri M. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res. 2014; 57:120-129.
21. Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, Miar A, Rodriguez-Garcia A, Tan DX, Reiter RJ, Mayo JC, Sainz RM. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res. 2015; 58:234-250.
22. Paroni R, Terraneo L, Bonomini F, Finati E, Virgili E, Bianciardi P, Favero G, Fraschini F, Reiter RJ, Rezzani R, Samaja M. Antitumour activity of melatonin in a mouse model of human prostate cancer: relationship with hypoxia signalling. J Pineal Res. 2014; 57:43-52.
23. Shiu SY, Leung WY, Tam CW, Liu VW, Yao KM. Melatonin MT1 receptor-induced transcriptional up-regulation of p27(Kip1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-kappaB): potential implications on prostate cancer chemoprevention and therapy. J Pineal Res. 2013; 54:69-79.
24. Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009; 47:8-14.
25. Ordonez R, Fernandez A, Prieto-Dominguez N, Martinez L, Garcia-Ruiz C, Fernandez-Checa JC, Mauriz JL, Gonzalez-Gallego J. Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J Pineal Res. 2015; 59:178-189.
26. Ordonez R, Carbajo-Pescador S, Prieto-Dominguez N, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014; 56:20-30.
27. Leon J, Casado J, Jimenez Ruiz SM, Zurita MS, Gonzalez-Puga C, Rejon JD, Gila A, Munoz de Rueda P, Pavon EJ, Reiter RJ, Ruiz-Extremera A, Salmeron J. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-kappabeta. J Pineal Res. 2014; 56:415-426.
28. Hong Y, Won J, Lee Y, Lee S, Park K, Chang KT, Hong Y. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J Pineal Res. 2014; 56:264-274.
29. Zhou Q, Gui S, Zhou Q, Wang Y. Melatonin inhibits the migration of human lung adenocarcinoma A549 cell lines involving JNK/MAPK pathway. PLoS One. 2014; 9:e101132.
30. Plaimee P, Khamphio M, Weerapreeyakul N, Barusrux S, Johns NP. Immunomodulatory effect of melatonin in SK-LU-1 human lung adenocarcinoma cells co-cultured with peripheral blood mononuclear cells. Cell Prolif. 2014; 47:406-415.
31. Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni GJ. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett. 2001; 22:45-47.
32. Mocchegiani E, Perissin L, Santarelli L, Tibaldi A, Zorzet S, Rapozzi V, Giacconi R, Bulian D, Giraldi T. Melatonin administration in tumor-bearing mice (intact and pinealectomized) in relation to stress, zinc, thymulin and IL-2. Int J Immunopharmacol. 1999; 21:27-46.
33. Lissoni P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol (Paris). 2007; 55:201-204.
34. Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991; 79(1-3):C153-158.
35. Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012; 52:139-166.
36. Eisenstein M. Chronobiology: stepping out of time. Nature. 2013; 497:S10-12.
37. de Bodinat C, Guardiola-Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010; 9:628-642.
38. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014; 64:207-218.
39. Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001; 21:6405-6412.
40. Glickman G, Levin R, Brainard GC. Ocular input for human melatonin regulation: relevance to breast cancer. Neuro Endocrinol Lett. 2002; 23 Suppl 2:17-22.
41. Gaddy JR, Rollag MD, Brainard GC. Pupil size regulation of threshold of light-induced melatonin suppression. J Clin Endocrinol Metab. 1993; 77:1398-1401.
42. Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. 1997; 12:537-546.
43. Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011; 96:E463-472.
44. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000; 526 Pt 3:695-702.
45. Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007; 13:303-328.
46. Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, Huang S, Chen J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013; 37:197-206.
47. Papantoniou K, Castano-Vinyals G, Espinosa A, Aragones N, Perez-Gomez B, Ardanaz E, Altzibar JM, Sanchez VM, Gomez-Acebo I, Llorca J, Munoz D, Tardon A, Peiro R, Marcos-Gragera R, Pollan M, Kogevinas M. Breast cancer risk and night shift work in a case-control study in a Spanish population. Eur J Epidemiol. 2015.
48. Akerstedt T, Knutsson A, Narusyte J, Svedberg P, Kecklund G, Alexanderson K. Night work and breast cancer in women: a Swedish cohort study. BMJ Open. 2015; 5:e008127.
49. Papantoniou K, Castano-Vinyals G, Espinosa A, Aragones N, Perez-Gomez B, Burgos J, Gomez-Acebo I, Llorca J, Peiro R, Jimenez-Moleon JJ, Arredondo F, Tardon A, Pollan M, Kogevinas M. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015; 137:1147-1157.
50. Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012; 176:751-759.
51. Wang X, Ji A, Zhu Y, Liang Z, Wu J, Li S, Meng S, Zheng X, Xie L. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015; 6:25046-25060. doi: 10.18632/oncotarget.4502.
52. Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, Gupta D, Watson K, Layer D, Huff-Adams S, Desai B, Sharma P, Wallam M, Delioukina M, Ball P, Bryant M, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005; 93:1202-1208.
53. Mazzoccoli G, Carughi S, De Cata A, La Viola M, Vendemiale G. Melatonin and cortisol serum levels in lung cancer patients at different stages of disease. Med Sci Monit. 2005; 11:Cr284-288.
54. Hu S, Shen G, Yin S, Xu W, Hu B. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Adv Ther. 2009; 26:886-892.
55. Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta. 2006; 1757(5-6):573-589.
56. Shah PN, Mhatre MC, Kothari LS. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 1984; 44:3403-3407.
57. Kothari LS. Influence of chronic melatonin on 9,10-dimethyl-1,2-benzanthracene-induced mammary tumors in female Holtzman rats exposed to continuous light. Oncology. 1987; 44:64-66.
58. Lenoir V, de Jonage-Canonico MB, Perrin MH, Martin A, Scholler R, Kerdelhue B. Preventive and curative effect of melatonin on mammary carcinogenesis induced by dimethylbenz[a]anthracene in the female Sprague-Dawley rat. Breast Cancer Res. 2005; 7:R470-476.
59. Dakshayani KB, Subramanian P, Manivasagam T, Essa MM, Manoharan S. Melatonin modulates the oxidant-antioxidant imbalance during N-nitrosodiethylamine induced hepatocarcinogenesis in rats. J Pharm Pharm Sci. 2005; 8:316-321.
60. Anisimov VN, Popovich IG, Zabezhinski MA. Melatonin and colon carcinogenesis: I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2-dimethylhydrazine in rats. Carcinogenesis. 1997; 18:1549-1553.
61. Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di S, Wu G, Yang Y, Reiter RJ, Ji G. Melatonin as a treatment for gastrointestinal cancer: a review. J Pineal Res. 2015; 58:375-387.
62. Anisimov VN, Zabezhinski MA, Popovich IG, Zaripova EA, Musatov SA, Andre V, Vigreux C, Godard T, Sichel F. Inhibitory effect of melatonin on 7, 12-dimethylbenz[a]anthracene-induced carcinogenesis of the uterine cervix and vagina in mice and mutagenesis in vitro. Cancer Lett. 2000; 156:199-205.
63. Vesnushkin GM, Plotnikova NA, Semenchenko AV, Anisimov VN. Melatonin inhibits urethane-induced carcinogenesis tumors in murine lung. Vopr Onkol. 2006; 52:164-168.
64. Biesalski HK, Bueno de Mesquita B, Chesson A, Chytil F, Grimble R, Hermus RJ, Kohrle J, Lotan R, Norpoth K, Pastorino U, Thurnham D. European Consensus Statement on Lung Cancer: risk factors and prevention. Lung Cancer Panel. CA Cancer J Clin. 1998; 48:167-176; discussion 164-166.
65. Smith CJ, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol. 2000; 38:637-646.
66. Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009; 9:377-384.
67. Ozguner F, Koyu A, Cesur G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health. 2005; 21(1-2):21-26.
68. El-Sokkary GH, Cuzzocrea S, Reiter RJ. Effect of chronic nicotine administration on the rat lung and liver: beneficial role of melatonin. Toxicology. 2007; 239(1-2):60-67.
69. Unlu M, Fidan F, Sezer M, Tetik L, Sahin O, Esme H, Koken T, Serteser M. Effects of melatonin on the oxidant/antioxidant status and lung histopathology in rabbits exposed to cigarette smoke. Respirology. 2006; 11:422-428.
70. Sun CK, Lee FY, Kao YH, Chiang HJ, Sung PH, Tsai TH, Lin YC, Leu S, Wu YC, Lu HI, Chen YL, Chung SY, Su HL, Yip HK. Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J Pineal Res. 2015; 58:137-150.
71. Shin IS, Shin NR, Park JW, Jeon CM, Hong JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR, Ahn KS. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res. 2015; 58:50-60.
72. Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001; 488:65-76.
73. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646-674.
74. Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000; 21:485-495.
75. Pariente R, Pariente JA, Rodriguez AB, Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016; 60:55-64.
76. Leja-Szpak A, Jaworek J, Pierzchalski P, Reiter RJ. Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J Pineal Res. 2010; 49:248-255.
77. Park EJ, Woo SM, Min KJ, Kwon TK. Transcriptional and post-translational regulation of Bim controls apoptosis in melatonin-treated human renal cancer Caki cells. J Pineal Res. 2014; 56:97-106.
78. Garcia-Santos G, Antolin I, Herrera F, Martin V, Rodriguez-Blanco J, del Pilar Carrera M, Rodriguez C. Melatonin induces apoptosis in human neuroblastoma cancer cells. J Pineal Res. 2006; 41:130-135.
79. Bizzarri M, Proietti S, Cucina A, Reiter RJ. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets. 2013; 17:1483-1496.
80. Fernandez A, Ordonez R, Reiter RJ, Gonzalez-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015; 59:292-307.
81. Vriend J, Reiter RJ. Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 2014; 115(1-2):8-14.
82. Jaworek J, Leja-Szpak A. Melatonin influences pancreatic cancerogenesis. Histol Histopathol. 2014; 29:423-431.
83. Rodriguez C, Martin V, Herrera F, Garcia-Santos G, Rodriguez-Blanco J, Casado-Zapico S, Sanchez-Sanchez AM, Suarez S, Puente-Moncada N, Anitua MJ, Antolin I. Mechanisms involved in the pro-apoptotic effect of melatonin in cancer cells. Int J Mol Sci. 2013; 14:6597-6613.
84. Proietti S, Cucina A, Reiter RJ, Bizzarri M. Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell Mol Life Sci. 2013; 70:2139-2157.
85. Sanchez-Hidalgo M, Guerrero JM, Villegas I, Packham G, de la Lastra CA. Melatonin, a natural programmed cell death inducer in cancer. Curr Med Chem. 2012; 19:3805-3821.
86. Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C. Melatonin: the smart killer: the human trophoblast as a model. Mol Cell Endocrinol. 2012; 348:1-11.
87. Mediavilla MD, Sanchez-Barcelo EJ, Tan DX, Manchester L, Reiter RJ. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem. 2010; 17:4462-4481.
88. Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003; 60:1407-1426.
89. Nasrabadi NN, Ataee R, Abediankenari S, Shokrzadeh M, Najafi M, Hoseini SV, Jan HH. Expression of MT2 receptor in patients with gastric adenocarcinoma and its relationship with clinicopathological features. J Gastrointest Cancer. 2014; 45:54-60.
90. Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, Liu L, Xie F, Kang T, Huang W, Deng W. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012; 53:77-90.
91. Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem Soc Trans. 2009; 37(Pt 3):605-613.
92. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003; 3:453-458.
93. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009; 9:274-284.
94. Thakur RK, Yadav VK, Kumar A, Singh A, Pal K, Hoeppner L, Saha D, Purohit G, Basundra R, Kar A, Halder R, Kumar P, Baral A, Kumar MJ, Baldi A, Vincenzi B, et al. Non-metastatic 2 (NME2)-mediated suppression of lung cancer metastasis involves transcriptional regulation of key cell adhesion factor vinculin. Nucleic Acids Res. 2014; 42:11589-11600.
95. Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000; 355:479-485.
96. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006; 127:679-695.
97. Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, Frasch T, Blask DE. Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer. 2015; 22:R183-204.
98. Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W, Chen YC, Jan YJ, Lai DW, Tang SC, Tien HR, Chiu CS, Tsai TC, Lai YL, Sheu ML. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPbeta and NFkappaB cleavage. J Pineal Res. 2016; 60:142-154.
99. Ortiz-Lopez L, Morales-Mulia S, Ramirez-Rodriguez G, Benitez-King G. ROCK-regulated cytoskeletal dynamics participate in the inhibitory effect of melatonin on cancer cell migration. J Pineal Res. 2009; 46:15-21.
100. Wang T, Liu G, Wang R. The Intercellular Metabolic Interplay between Tumor and Immune Cells. Front Immunol. 2014; 5:358.
101. Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006; 90:1-50.
102. June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007; 117:1466-1476.
103. Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013; 13:525-541.
104. Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. 2013; 14:8638-8683.
105. Calvo JR, Gonzalez-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J Pineal Res. 2013; 55:103-120.
106. Guerrero JM, Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem. 2002; 2:167-179.
107. Espino J, Pariente JA, Rodriguez AB. Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid Med Cell Longev. 2012; 2012:670294.
108. Sainz RM, Mayo JC, Uria H, Kotler M, Antolin I, Rodriguez C, Menendez-Pelaez A. The pineal neurohormone melatonin prevents in vivo and in vitro apoptosis in thymocytes. J Pineal Res. 1995; 19:178-188.
109. Hoijman E, Rocha Viegas L, Keller Sarmiento MI, Rosenstein RE, Pecci A. Involvement of Bax protein in the prevention of glucocorticoid-induced thymocytes apoptosis by melatonin. Endocrinology. 2004; 145:418-425.
110. Tian YM, Zhang GY, Dai YR. Melatonin rejuvenates degenerated thymus and redresses peripheral immune functions in aged mice. Immunol Lett. 2003; 88:101-104.
111. Provinciali M, Di Stefano G, Bulian D, Tibaldi A, Fabris N. Effect of melatonin and pineal grafting on thymocyte apoptosis in aging mice. Mech Ageing Dev. 1996; 90:1-19.
112. Tian YM, Li PP, Jiang XF, Zhang GY, Dai YR. Rejuvenation of degenerative thymus by oral melatonin administration and the antagonistic action of melatonin against hydroxyl radical-induced apoptosis of cultured thymocytes in mice. J Pineal Res. 2001; 31:214-221.
113. Maestroni GJ, Conti A, Lissoni P. Colony-stimulating activity and hematopoietic rescue from cancer chemotherapy compounds are induced by melatonin via endogenous interleukin 4. Cancer Res. 1994; 54:4740-4743.
114. Maestroni GJ, Covacci V, Conti A. Hematopoietic rescue via T-cell-dependent, endogenous granulocyte-macrophage colony-stimulating factor induced by the pineal neurohormone melatonin in tumor-bearing mice. Cancer Res. 1994; 54:2429-2432.
115. Currier NL, Sun LZ, Miller SC. Exogenous melatonin: quantitative enhancement in vivo of cells mediating non-specific immunity. J Neuroimmunol. 2000; 104:101-108.
116. Arias J, Melean E, Valero N, Pons H, Chacin-Bonilla L, Larreal Y, Bonilla E. Effect of melatonin on lymphocyte proliferation and production of interleukin-2 (IL-2) and interleukin-1 beta (IL-1 beta) in mice splenocytes. Invest Clin. 2003; 44:41-50.
117. Garcia-Maurino S, Gonzalez-Haba MG, Calvo JR, Goberna R, Guerrero JM. Involvement of nuclear binding sites for melatonin in the regulation of IL-2 and IL-6 production by human blood mononuclear cells. J Neuroimmunol. 1998; 92(1-2):76-84.
118. Garcia-Maurino S, Gonzalez-Haba MG, Calvo JR, Rafii-El-Idrissi M, Sanchez-Margalet V, Goberna R, Guerrero JM. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: a possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J Immunol. 1997; 159:574-581.
119. Garcia-Maurino S, Pozo D, Carrillo-Vico A, Calvo JR, Guerrero JM. Melatonin activates Th1 lymphocytes by increasing IL-12 production. Life Sci. 1999; 65:2143-2150.
120. Vijayalaxmi, Reiter RJ, Tan DX, Herman TS, Thomas CR, Jr. Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004; 59:639-653.
121. Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol Metab. 1977; 45:768-774.
122. Kontek R, Nowicka H. The modulatory effect of melatonin on genotoxicity of irinotecan in healthy human lymphocytes and cancer cells. Drug Chem Toxicol. 2013; 36:335-342.
123. Norsa A, Martino V. Somatostatin, retinoids, melatonin, vitamin D, bromocriptine, and cyclophosphamide in chemotherapy-pretreated patients with advanced lung adenocarcinoma and low performance status. Cancer Biother Radiopharm. 2007; 22:50-55.
124. Fic M, Podhorska-Okolow M, Dziegiel P, Gebarowska E, Wysocka T, Drag-Zalesinska M, Zabel M. Effect of melatonin on cytotoxicity of doxorubicin toward selected cell lines (human keratinocytes, lung cancer cell line A-549, laryngeal cancer cell line Hep-2). In Vivo. 2007; 21:513-518.
125. Lu JJ, Fu L, Tang Z, Zhang C, Qin L, Wang J, Yu Z, Shi D, Xiao X, Xie F, Huang W, Deng W. Melatonin inhibits AP-2beta/hTERT, NF-kappaB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget. 2016; 7:2985-3001. doi: 10.18632/oncotarget.6407.
126. Fumagalli L, Lissoni P, Di Felice G, Meregalli S, Valsuani G, Mengo S, Rovelli F. Pretreatment serum markers and lymphocyte response to interleukin-2 therapy. Br J Cancer. 1999; 80(3-4):407-411.
127. Lissoni P, Brivio F, Fumagalli L, Messina G, Vigore L, Parolini D, Colciago M, Rovelli F. Neuroimmunomodulation in medical oncology: application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Res. 2008; 28(2b):1377-1381.
128. Lissoni P, Barni S, Mandala M, Ardizzoia A, Paolorossi F, Vaghi M, Longarini R, Malugani F, Tancini G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999; 35:1688-1692.
129. Lissoni P, Paolorossi F, Ardizzoia A, Barni S, Chilelli M, Mancuso M, Tancini G, Conti A, Maestroni GJ. A randomized study of chemotherapy with cisplatin plus etoposide versus chemoendocrine therapy with cisplatin, etoposide and the pineal hormone melatonin as a first-line treatment of advanced non-small cell lung cancer patients in a poor clinical state. J Pineal Res. 1997; 23:15-19.
130. Sookprasert A, Johns NP, Phunmanee A, Pongthai P, Cheawchanwattana A, Johns J, Konsil J, Plaimee P, Porasuphatana S, Jitpimolmard S. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014; 34:7327-7337.
131. Lissoni P, Tancini G, Barni S, Paolorossi F, Ardizzoia A, Conti A, Maestroni G. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Support Care Cancer. 1997; 5:126-129.
132. Del Fabbro E, Dev R, Hui D, Palmer L, Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol. 2013; 31:1271-1276.
133. Yun M, Kim EO, Lee D, Kim JH, Kim J, Lee H, Lee J, Kim SH. Melatonin sensitizes H1975 non-small-cell lung cancer cells harboring a T790M-targeted epidermal growth factor receptor mutation to the tyrosine kinase inhibitor gefitinib. Cell Physiol Biochem. 2014; 34:865-872.
134. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005; 352:786-792.
135. Steuer CE, Ramalingam SS. Targeting EGFR in lung cancer: Lessons learned and future perspectives. Mol Aspects Med. 2015; 45:67-73.
136. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.
137. Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S, Jr., Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22:330-353.
138. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997; 15:2996-3018.
139. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocrine J. 1993; 1:57-60.
140. Mihandoost E, Shirazi A. Can melatonin help us in radiation oncology treatments? 2014; 2014:578137.
141. Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000; 225:9-22.
142. Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda). 2014; 29:325-333.
143. Jang SS, Kim HG, Lee JS, Han JM, Park HJ, Huh GJ, Son CG. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression. Int J Radiat Biol. 2013; 89:97-105.
144. Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, Naghizadeh MM, Ghasemi D, Keshavarz M, Haddadi GH. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015; 17:111-120.
145. Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS, Kumar KS. Melatonin and protection from whole-body irradiation: survival studies in mice. Mutat Res. 1999; 425:21-27.
146. Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, Chen YC, Honoki K, Fujii H, Georgakilas AG, Nowsheen S, Amedei A, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015; 35 Suppl:S224-243.
147. Cao Y. VEGF-targeted cancer therapeutics-paradoxical effects in endocrine organs. Nat Rev Endocrinol. 2014; 10:530-539.
148. Dai M, Cui P, Yu M, Han J, Li H, Xiu R. Melatonin modulates the expression of VEGF and HIF-1 alpha induced by CoCl2 in cultured cancer cells. J Pineal Res. 2008; 44:121-126.
149. Jardim-Perassi BV, Lourenco MR, Doho GM, Grigolo IH, Gelaleti GB, Ferreira LC, Borin TF, Moschetta MG, Pires de Campos Zuccari DA. Melatonin Regulates Angiogenic Factors under Hypoxia in Breast Cancer Cell Lines. Anticancer Agents Med Chem. 2016; 16:347-358.
150. Sohn EJ, Won G, Lee J, Lee S, Kim SH. Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells. J Cancer. 2015; 6:19-28.
151. Cho SY, Lee HJ, Jeong SJ, Lee HJ, Kim HS, Chen CY, Lee EO, Kim SH. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1alpha inactivation in hypoxic PC-3 prostate cancer cells. J Pineal Res. 2011; 51:87-93.
152. Park JW, Hwang MS, Suh SI, Baek WK. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J Pineal Res. 2009; 46:415-421.
153. Wang RX, Liu H, Xu L, Zhang H, Zhou RX. Involvement of nuclear receptor RZR/RORgamma in melatonin-induced HIF-1alpha inactivation in SGC-7901 human gastric cancer cells. Oncol Rep. 2015; 34:2541-2546.
154. Park SY, Jang WJ, Yi EY, Jang JY, Jung Y, Jeong JW, Kim YJ. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res. 2010; 48:178-184.
155. Carbajo-Pescador S, Ordonez R, Benet M, Jover R, Garcia-Palomo A, Mauriz JL, Gonzalez-Gallego J. Inhibition of VEGF expression through blockade of Hif1alpha and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 2013; 109:83-91.
156. Lv D, Cui PL, Yao SW, Xu YQ, Yang ZX. Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells. Chin J Cancer Res. 2012; 24:310-316.
157. Cui P, Yu M, Peng X, Dong L, Yang Z. Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J Pineal Res. 2012; 52:236-243.
158. Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004; 4:839-849.
159. Bremnes RM, Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund LT. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011; 6:209-217.
160. Kim GD, Lee SE, Kim TH, Jin YH, Park YS, Park CS. Melatonin suppresses acrolein-induced IL-8 production in human pulmonary fibroblasts. J Pineal Res. 2012; 52:356-364.
161. Massague J. TGFbeta in Cancer. Cell. 2008; 134:215-230.
162. Wang W, Li Q, Yamada T, Matsumoto K, Matsumoto I, Oda M, Watanabe G, Kayano Y, Nishioka Y, Sone S, Yano S. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009; 15:6630-6638.
163. El-Nikhely N, Larzabal L, Seeger W, Calvo A, Savai R. Tumor-stromal interactions in lung cancer: novel candidate targets for therapeutic intervention. Expert Opin Investig Drugs. 2012; 21:1107-1122.
164. Anisimov VN, Vinogradova IA, Panchenko AV, Popovich IG, Zabezhinski MA. Light-at-night-induced circadian disruption, cancer and aging. Curr Aging Sci. 2012; 5:170-177.
165. Kannen V, Marini T, Zanette DL, Frajacomo FT, Silva GE, Silva WA, Jr., Garcia SB. The melatonin action on stromal stem cells within pericryptal area in colon cancer model under constant light. Biochem Biophys Res Commun. 2011; 405:593-598.
166. Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, Rueda N. Breast cancer therapy based on melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2012; 6:108-116
Gerelateerde artikelen
- Wat is melatonine. Een inleiding op de artikelen over melatonine door arts-bioloog drs. E. Valstar.
- Melatonine geeft op 5-jaars meting 25 procent meer ziektevrije overleving bij patienten met operabele niet-kleincellige longkanker stadium III/IV in vergelijking met placebo
- Melatonine vermindert pijn bij patienten met borstkanker die chemo krijgen en vermindert ontstaan depressieve symptomen na operatie van borstkanker
- Melatonine - een referentielijst van studies met melatonine
- Melatonine geeft significant betere kansen op overleving, minder kans op recidief en vermindert hoog significant bijwerkingen bij chemo en bestraling - radiotherapie, aldus meta analyse van tientallen gerandomiseerde studies
- Melatonine geeft zowel als aanvullend middel bij een behandeling van kanker als preventief om kanker te voorkomen uitstekende resultaten blijkt uit 2 grote reviewstudies
- Algemeen: Melatonine bij patiënten met solide tumoren in vergevorderd stadium uitgezonderd melanomen en niercarcinomen welke behandeld worden met interleukine-2.
- Algemeen: Melatoninewaarden door lage dosis interleukine-2 toegevoegd aan gerichte psychosociale begeleiding bij patienten met vergevorderde kanker en solide tumoren geeft significant betere kwaliteit van leven en langere overlevingstijd.
- Borstkanker -: s' Nachts werken en gebrek aan zonlicht lijkt een verhoogd risico voor ontwikkelen van borstkanker aldus enkele grote studies. Mogelijke oorzaak lijkt een gebrek aan melatonine aanmaak.
- Borstkanker: Melatonine naast Nolvadex - Tamoxifen bij vergevorderde borstkanker welke hormoonongevoelig zijn. Een studie analyse van arts-bioloog drs. E. Valstar.
- Borstkanker - Melatonine waarden lijken voorspellers van risico op borstkanker. Wie regelmatig goed slaapt 's nachts heeft hogere melatonine waarden in ochtend urine dan wie niet goed in het donker slaapt of vaker overdag slaapt i.v.m. nachtdienst
- Borstkanker - Lang en goed slapen 's nachts lijkt beschermend tegen borstkanker. Echte langslapers hadden beduidend minder kans op borstkanker dan kort slapers. Dit alles lijkt te maken te hebben met natuurlijke melatonine aanmaak
- Darmkanker - s' Nachts werken en gebrek aan zonlicht lijkt een verhoogd risico voor ontwikkelen van darmkanker aldus studie uit Pubmed. Mogelijke oorzaak lijkt een gebrek aan melatonine aanmaak.
- Longkanker - Melatonine verlengt leven bij patiënten met uitgezaaide niet kleincellige longkanker. Een bespreking van twee studies door drs. E. Valstar
- Darmkanker - Melatonine bij uitgezaaide darmkanker, een studiebespreking van Drs. E. Valstar.
- Hersenmetastases (uitzaaiingen) van solide tumoren en effect van melatonine daarbij. Melatonine bij hersenmetastasen van solide tumoren en bij vergevorderde borstkanker.
- Levertumoren: melatonine, vooraf aan TACE geeft significant - 16 procent - meer 2-jaars overlevingen dan alleen met TACE en significant minder bijwerkingen en significant beter leverfunctioneren werd klinisch aangetoond in studie met 100 patiënten
- Longkanker: Melatonine verlengt leven significant wanneer consequent gegeven naast chemokuren bij niet-kleincellige longkanker en darmkanker.
- Longkanker: Melatonine en 5-MTT = 5-methoxytryptamine, verbetert significant de resultaten en vermindert significant de bijwerkingen wanneer gegeven naast chemokuren voor niet-kleincellige longkanker.
- Longkanker: Melatonine naast chemo - cisplatin en etoposide - zorgt voor significant langer leven met betere kwaliteit van leven, aldus gerandomiseerde 5-jarige studie met 100 longkankerpatienten
- Melanomen: Melatonine als therapeuticum bij het maligne melanoom en astrocytomen graad 3/4.
- Prostaatkanker: Melatonine aangemaakt door een goede nachtrust beschermt tegen prostaatkanker. Het risico op het krijgen van prostaatkanker nam met 75% af
- Spijsverteringskanker: Melatonine aanmaak, melatonine tekort en melatonine suppletie spelen grote rol bij ons immuunsysteem en preventie en behandelen van spijsverteringskanker waaronder slokdarmkanker
- Melatonine, een overzicht van artikelen over melatonine als aanvulling bij een kankerbehandeling bij elkaar gezet.



Plaats een reactie ...
Reageer op "Melatonine geeft significant betere kansen op overleving, minder kans op recidief en vermindert hoog significant bijwerkingen bij chemo en bestraling - radiotherapie, aldus meta analyse van tientallen gerandomiseerde studies"