Onze IBANcode is NL79 RABO 0372 9311 38 BIC/SWIFTCODE RABONL2U
En wij hebben een ANBI status dus uw donatie is in principe aftrekbaar. En als donateur kunt u ook korting krijgen bij verschillende bedrijven.
21 oktober 2022: Bron: Blood 7 th of july 2022 en Nature
Uit een fase I studie bij oorspronkelijk 29 patiënten van 2 tot 70 jaar met vergevorderde B-cell acute lymfoblastische leukemia (B-ALL) waarvan er 20 patiënten werden geanalyseerd blijkt dat immuuntherapie met de zogeheten FasT CAR-T celtherapie (F-CAR-T) uitstekende resultaten geeft.
Van de 20 patiénten die werden geanalyseerd blijken er na 2 jaar 15 een langdurige complete remissie te hebben bereikt van mediaan 725 dagen op moment van de analyse.
1 patiënt kreeg uiteindelijk binnen de studieduur een recidief. 4 patiënten overleden als gevolg van de bijwerkingen van de allogene stamceltransplantatie die werd gedaan. CD19 F-CAR-T-cellen vertoonden uitstekende proliferatie met een jonger cellulair fenotype, minder uitputting van de T-cellen en effectievere tumoreliminatie in vergelijking met conventionele CAR-T-cellen in deze Fase I studie.
Het veiligheidsprofiel van CD19 F-CAR-T was beheersbaar met 24% graad 3 cytokine release syndrome (CRS) en 28% graad 3/4 neurotoxiciteit die voornamelijk bij pediatrische patiënten voorkwam. Op dag 14 bereikten 23/25 patiënten minimale residuele ziekte (MRD)-negatieve complete remissie (CR), en 20 ondergingen vervolgens allogene hematopoëtische stamceltransplantatie (allo-HSCT) binnen 3 maanden na F-CAR-T-therapie. Van de drie patiënten die geen allo-HSCT ondergingen, bleven er twee in CR tot 10 maanden na F-CAR-T.
FasT CAR-T celtherapie (F-CAR-T) is een vorm van CAR-T celtherapie waarbij de CAR-T cellen veel sneller aan de patiënt kan worden gegeven in vergelijking met de standaard CAR-T celtherapie. Voor gewone CAR-T cellen duurt het meestal een maand voordat deze aan de patiënt kunnen worden gegeven. Bij de FasT CAR-T celtherapie kan dat in principe direct de volgende dag. Al duurde het in deze studie nog 7 dagen voor aan alle eisen was voldaan.
De vrijgave criteria zijn samengevat in Tabel S1. Kortom, de productietijd voor F-CAR-T was de volgende dag plus nog ongeveer 7 dagen QC-tests en extra tijd voor vrijgave en transport van cellen (Fig. S1B). Maar die tijd tussen het geven van de CAR-T cellen en de stamtransplantatie is dus heel belangrijk. Hoe korter die tussentijd hoe beter.
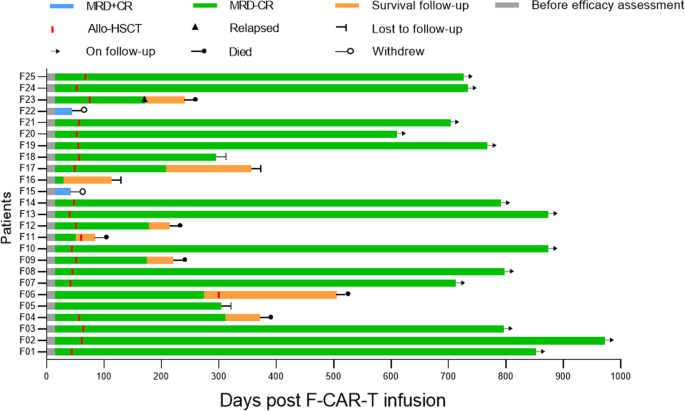
Clinical outcomes and consolidative allo-HSCT for the 25 patients who were treated with F-CAR-T therapy are shown. On day 28, 23/25 patients achieved MRD-negative CR/CRi. With a median time of 54 days (range: 45–81) post F-CAR-T infusion, 20 of 23 patients with MRD-negative status received consolidative allo-HSCT. Among the 20 patients, 1 patient (F23) relapsed on day 172 and died 3 months after relapse. Four patients (F04, F09, F11, F12) died from transplant-related mortality (TRM) including infection (n = 3) and chronic GVHD (n = 1) on day 84, day 215, day 220, and day 312, respectively. The remaining 15 patients were in MRD-negative CR except for one (F18) who became MRD-positive on day 294. Among the other 3 patients (F05, F06, F16), 1 remained MRD-negative CR on day 304, 1 remained in MRD-negative CR until day 303, received allo-HSCT, and subsequently died from an infection on day 505. One patient was lost to follow-up after day 114. MRD minimal residual disease, CR complete remission, Allo-HSCT allogeneic hematopoietic stem cell transplantation.
Hier de originele Engelse tekst uit de introductie met referentieverwijzingen, daaronder het abstract:
Introduction
CD19-targeting chimeric antigen receptor-engineered T cells (CAR-T) represent a major advancement in refractory/relapsed (R/R) B-cell acute lymphocytic leukemia (B-ALL) with high initial complete remission (CR) rate of around 70–90% [1,2,3,4,5,6,7,8]. However, current CAR-T cell manufacturing requires a long waiting time for patients, typically requiring a minimum of 7–14 days of manufacturing time [9,10,11,12].
Two recent large-scale CD19-targeted CAR-T clinical trials [9, 11] reported that 20–30% of enrolled patients with B-ALL ultimately were not infused with CAR-T cells due to death from rapid disease progression or CAR-T cell manufacturing failure. Thus, shortening the duration between apheresis and CAR-T infusion is critical for patients with R/R B-ALL. Furthermore, the high cost of commercially available CAR-T cell products creates a major access barrier and limits its broad application for patients who could benefit from this novel therapeutic technology.
Notably, about 28–43% of B-ALL patients who achieve CR after CAR-T cell treatment relapse, highlighting the importance of optimizing the duration of remission after CAR-T treatment. Prolonged ex vivo culture and expansion of T- cells are associated with reduced lifespan and potency of CAR-T after adoptive transfer [13,14,15,16,17]. A recent study [18] reported that CAR-T cells manufactured within 3 days exerted enhanced proliferative capacity and increased anti-leukemia as compared to those produced within 5 or 9 days in a preclinical study. However, the feasibility of accelerated CAR-T production and its efficacy and potency in the clinic have not been widely tested [9,10,11].
To shorten the manufacturing time, minimize the cost, and optimize the function of CAR-T cell therapy, the novel anti-CD19 CAR-T therapy called FasT CAR-T (F-CAR-T) was developed with a significantly expedited CAR-T next-day manufacturing process. Here, we describe the preclinical and phase I clinical study of CD19 F-CAR-T therapy in R/R B-ALL patients.
- Article
- Open Access
- Published:
Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study
Blood Cancer Journal volume 12, Article number: 104 (2022)
Abstract
To improve clinical outcomes and shorten the vein-to-vein time of chimeric antigen receptor T (CAR-T) cells, we developed the FasT CAR-T (F-CAR-T) next-day manufacturing platform. We report the preclinical and first-in-human clinical studies evaluating the safety, feasibility, and preliminary efficacy of CD19 F-CAR-T in B-cell acute lymphoblastic leukemia (B-ALL). CD19 F-CAR-T cells demonstrated excellent proliferation with a younger cellular phenotype, less exhaustion, and more effective tumor elimination compared to conventional CAR-T cells in the preclinical study. In our phase I study (NCT03825718), F-CAR-T cells were successfully manufactured and infused in all of the 25 enrolled pediatric and adult patients with B-ALL. CD19 F-CAR-T safety profile was manageable with 24% grade 3 cytokine release syndrome (CRS) and 28% grade 3/4 neurotoxicity occurring predominantly in pediatric patients. On day 14, 23/25 patients achieved minimal residual disease (MRD)-negative complete remission (CR), and 20 subsequently underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) within 3 months post F-CAR-T therapy. Fifteen of 20 patients were disease-free with a median remission duration of 734 days. One patient relapsed and 4/20 died from transplant-related mortality. Of the three patients who did not undergo allo-HSCT, two remained in CR until 10 months post-F-CAR-T. Our data indicate that anti-CD19 FasT CAR-T shows promising early efficacy for B-ALL. Further evaluations in larger clinical studies are needed.
Data availability
To get a detailed protocol on the clinical trial, please find it at https://clinicaltrials.gov, NCT03825718. For any information regarding this manuscript, please contact the corresponding author at peihua_lu@126.com.
References
-
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood, 2016;127:3312–20.
-
Ceppi F, Gardner RA. Chimeric Antigen receptor T cells for B-cell acute lymphoblastic leukemia. Cancer J. 2019;25:191–8.
-
Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–76.
-
Frey NV. Chimeric antigen receptor T cells for acute lymphoblastic leukemia. Am J Hematol. 2019;94:S24–S27.
-
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224–5.
-
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl J Med. 2014;371:1507–17. https://doi.org/10.1056/NEJMoa1407222.
-
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
-
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38.
-
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl J Med. 2018;378:449–59.
-
Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–31.
-
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
-
Tumaini B, Lee DW, Lin T, Castiello L, Stroncek DF, Mackall C, et al. Simplified process for the production of anti-CD19-CAR-engineered T cells. Cytotherapy 2013;15:1406–15.
-
Dolnikov A, Shen S, Klamer G, Joshi S, Xu N, Yang L, et al. Antileukemic potency of CD19-specific T cells against chemoresistant pediatric acute lymphoblastic leukemia. Exp Hematol. 2015;43:1001–1014.e5.
-
Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7.
-
Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–84.
-
Wang X, Wong CW, Urak R, Taus E, Aguilar B, Chang WC, et al. Comparison of naive and central memory derived CD8+ effector cell engraftment fitness and function following adoptive transfer. Oncoimmunology. 2016;5:e1072671.
-
Durek P, Nordström K, Gasparoni G, Salhab A, Kressler C, de Almeida M, et al. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity. 2016;45:1148–61.
-
Ghassemi S, Nunez-Cruz S, O’Connor RS, Fraietta JA, Patel PR, Scholler J, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol Res. 2018;6:1100–9.
-
Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 2018;53:164–81. https://doi.org/10.1016/j.copbio.2018.01.025.
-
Zhang X, Lu XA, Yang J, Zhang G, Li J, Song L, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020;4:2325–38. https://doi.org/10.1182/bloodadvances.2020001466.
-
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133:1652–63.
-
Jiang H, Li C, Yin P, Guo T, Liu L, Xia L, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am J Hematol. 2019;94:1113–22.
-
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
-
National Cancer Institute. Common terminology criteria for adverse events (CTCAE). Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf; 2018.
-
Chaix J, Nish SA, Lin WH, Rothman NJ, Ding L, Wherry EJ, et al. Cutting edge: CXCR4 is critical for CD8+ memory T cell homeostatic self-renewal but not rechallenge self-renewal. J Immunol. 2014;193:1013–6. https://doi.org/10.4049/jimmunol.1400488.
-
Goedhart M, Gessel S, van der Voort R, Slot E, Lucas B, Gielen E, et al. CXCR4, but not CXCR3, drives CD8+ T-cell entry into and migration through the murine bone marrow. Eur J Immunol. 2019;49:576–89.
-
Zhang X, Yang J, Li J, Li W, Song D, Lu XA, et al. Factors associated with treatment response to CD19 CAR-T therapy among a large cohort of B cell acute lymphoblastic leukemia. Cancer Immunol Immunother. 2021. https://doi.org/10.1007/s00262-021-03009-z.
-
Liu S, Deng B, Yin Z, Pan J, Lin Y, Ling Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10:15.
-
Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134:2149–58.
-
Rouce RH. The earlier the better: timely mitigation of CRS. Blood. 2019;134:2119–20.
-
Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Lyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137:3272–6. https://doi.org/10.1182/blood.2020008865.
-
Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–19.
-
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. https://doi.org/10.1158/2159-8290.CD-16-0040.
-
Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4.
-
Yang J, Jiang P, Zhang X, Li J, Wu Y, Xu L, et al. Successful 24-hours manufacture of Anti-CD19/CD22 dual chimeric antigen receptor (CAR) T cell therapy for B-cell acute lymphoblastic leukemia (B-ALL). Blood. 2020;136:2–3. https://doi.org/10.1182/blood-2020-136866.
Acknowledgements
We are indebted to the courageous patients who participated in this study and their families. We greatly appreciate the staff in the laboratory for immunotherapy from Lu Daopei Hospital and Gracell who performed the CAR-T-related tests and cytokine analyses, and the physicians in the department of HSCT for providing clinical consultation and care, and all the nurses for their devotion and patient care. We want to thank Gracell Biotechnologies Co., Ltd and their staff that provided the CAR-T products.
Author information
Authors and Affiliations
Contributions
PL, JY, XZ and Gracell Biotechnologies Clinical science department designed the clinical study. PL, JY and XZ conducted the clinical study and provided patient care. JH is a major contributor in developing the Fast CAR-T manufacturing platform. LS, YZ and WY are the major contributors in the preclinical studies. QW recorded the case report form. ZS is a major contributor in manufacturing F-CAR-T cell product. LS, XY and LQ performed the flow cytometry, qPCR and cytokine tests. ZW and JL are major contributors in analyzing clinical data. PL, SM, LS, JY, XZ, JL, WC and ZW analyzed the data, wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
JH, WC, QW, ZS, WY, LS, and MS are employees of Gracell Biotechnologies Co., Ltd. YZ, ZW, and XY were employees of Gracell Biotechnologies Co., Ltd. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Gerelateerde artikelen
- Wat houdt CAR-T celtherapie in? Zie overzicht en stand van zaken. Met extra aandacht voor CAR-T celt therapie bij Acute Myeloide Leukemie
- CAR-T celtherapie gericht op de CLL1 en CD33 expressie met dubbele aanpak geeft hoopvolle resultaten bij Acute Myeloide Leukemie. Een vrouw met recidief van AML na beenmergtransplantatie komt zelfs in een duurzame complete remissie
- CAR-T-celtherapie geneest patienten met Lupus en leukemie maar is duur, ziekmakende afweercellen wegzuigen uit bloed van proefdieren via mRNA is even effectief en goedkoper
- CAR-T celtherapie maakt dat kinderen met neuroblastoma nu in 2025 al meer dan 10 jaar kankervrij zijn. Met uitgelicht een prachtig overlevingsverhaal van een kind van 4 jaar die anno 2025 al 19 jaar kankervrij is.
- CD19 Fast-CAR-T-cel therapie bij patienten met vergevorderde B-cel acute lymfatische leukemie (B-ALL) geven hele goede resultaten bij 15 van de 20 patienten langdurige complete remissies
- CAR-T celtherapie brengt binnen 1 jaar al 7 van de 44 patienten met leukemie en lymfklierkanker in totale remissie in Spanje in een open gezondheidsprogramma
- CAR-T celtherapie is zeer succesvol bij kankerpatienten maar loopt in Nederland vast op te strenge milieueisen, stellen Nederlandse top wetenschappers copy 1
- Immuuntherapie met gemanipuleerde T-cellen geeft spectaculair goede resultaten bij patienten met vergevorderde leukemie en B-cel lymfomen
- Immuuntherapie met gemanipuleerde T-cellen - CAR-T celtherapie ( tisagenlecleucel ) geeft spectaculair goede resultaten bij patienten met gevorderde lymfklierkanker - non-Hodgkin (B-lymfomen)
- Dendritische celtherapie met T-car cells wordt als proef vergoed vanuit basiszorgverzekering voor melanomen stadium IIIB en IIIC
- Longkanker: Dendritische celtherapie plus gemoduleerde T-cellen naast chemo geeft 25 procent betere mediane overall overleving op 5 jaar bij operabele niet-kleincellige longkanker
- Immuuntherapie met TIL - tumor infiltrating lymfocyten zorgt bij een kwart van de deelnemers voor jarenlange ziektevrije tijd bij patienten met uitgezaaide melanomen
- Immuuntherapie met CAR T-Cell Therapy (tisagenlecleucel (Kymriah) goedgekeurd door FDA voor gebruik bij kinderen en jong volwassenen met vorm van Acute Lymfatische Leukemie (ALL). copy 1 copy 1
- Immuuntherapie met T-car cells - Tumor Lymphocytic Infiltration is interessante ontwikkeling bij verschillende vormen van kanker. Hoe meer TLI hoe beter
- Immuuntherapie met gemoduleerde T-car cells geeft bij zwaar voorbehandelde lymfklierkanker non-Hodgkin alsnog uitstekende resultaten met 33 en 50 procent complete remissies
- Receptor-gemodificeerde T-cellen leiden tot totale remissies bij uitbehandelde agressieve acute lymfatische leukemie - ALL en geven hoop op genezende behandeling. copy 1
- Autologe genetisch gemodificeerde T-cellen gericht tegen het humaan papillomavirus (HPV) 16 E6 geeft bij patienten met vergevorderde zwaar voorbehandelde uitgezaaide HPV gerelateerde kanker uitstekende resultaten
- CAR-T cel therapie is een vorm van immuuntherapie die hele goede resultaten geeft. In Jama een overzicht van stand van zaken in de klinische praktijk



Plaats een reactie ...
Reageer op "CD19 Fast-CAR-T-cel therapie bij patienten met vergevorderde B-cel acute lymfatische leukemie (B-ALL) geven hele goede resultaten bij 15 van de 20 patienten langdurige complete remissies"