Helpt u ons aan 500 donateurs?
19 mei 2018: lees ook dit artikel:
31 augustus 2017: Lees ook dit artikel:
7 november 2015: lees het verhaal van Layla, een baby nog geen jaar oud die via immuuntherapie met T-cellen van een donor, T-cell enginering geheten, toegepast vlak voordat ze zou sterven, alsnog in een totale remissie kwam en nu al ruim dire maanden kankervrij is. Het verhaal van Layla met All - Acute Lymfatische Leukemie is wereldwijd overal gepubliceerd maar lees hier hoe de Volkskrant en Nature erover schrijven:
28 november 2016: kijk naar Nieuwsuur: http://nos.nl/nieuwsuur/artikel/2145326-vaccin-tegen-huidkanker-voor-meer-mensen-beschikbaar.html?title=vaccin-tegen-huidkanker-voor-meer-mensen-beschikbaar
en in het NPO journaal d.d. 28 november 2016: http://www.npo.nl/live/npo-1
het item met Jolanda de Vries over immuuntherapie met dendritische celtherapie bij melanomen, wat wel wat anders gaat dan met T-CAR cells (TIL - Tumor Infiltrating Lymfocyten) zoals hieronder beschreven maar is wel heel interessant. joalanda was ook bij ons overleg over utopie of uitdaging afgelopen woensdag 23 november 2016
31 maart 2016: Bron: VWS
Gaat het dan nu eindelijk gebeuren? Het is nog maar een heel klein begin maar wel hoopgevend dat Minister Schippers dendritische celtherapie met T-CAR cells (TIL - Tumor Infiltrating Lymfocyten) bij melanomen in stadium IIIB en IIIC als proef gaat vergoeden vanuit het basispakket per 1 april 2016.
Het is een heel bescheiden begin want als je dit studierapport leest: CAR-modified T-cell therapy for cancer: an updated review met een update van dendritische celtherapie met TIL - Tumor Infiltrating Lymfocyten bij veel verschillende vormen van kanker dan zou deze vorm van behandelen toch al lang moeten worden toegepast als studie in een veel eerder stadium van de ziekte en bij nagenoeg elke vorm van kanker lijkt mij. Want eigenljk altijd zijn de resultaten positief en dat bij vaak zwaar voorbehandelede kankerpatiënten. Zie ook referentielijst onderaan dit artikel
Zie ook deze artikelen over immuuntherapie met TIL - tumor infiltrating Lymfocyten:
en TILL bij Acute Lymfatische Leukemie - ALL:
en TILL bij bepaalde vorm van leukemie en lymfklierkanker:
Dit persbericht ontving ik vanmorgen in mijn mailbox:
Immuuntherapie tegen huidkanker voorwaardelijk toegelaten tot het basispakket
Minister Edith Schippers (VWS) laat de huidkankerbehandeling Dendritische Celtherapie voorwaardelijk toe tot het basispakket van de zorgverzekering. De vergoeding gaat in op 1 april 2016. Dat schrijft zij vandaag in een brief aan de Tweede Kamer.
Celtherapie
Dendritische celtherapie is een experimentele behandeling waarbij de patiënt een vaccin krijgt toegediend dat zijn immuunsysteem stimuleert om kankercellen op te ruimen.
Vergoeding
De vergoeding van dendritische celtherapie geldt voor de duur van vijf jaar en vier maanden. De therapie wordt vergoed voor patiënten met stadium IIIB en IIIC melanoom (specifieke vorm van huidkanker) na complete resectie (medische uitsnijding). Voor deze voorwaardelijke toelating is een budget van 19,9 miljoen euro beschikbaar.
Voorwaardelijke toelating
In 2012 werd voor het eerst een behandeling voorwaardelijk toegelaten. Door behandelingen en medicijnen voorwaardelijk tot het verzekerde pakket toe te laten krijgen patiënten toegang tot deze veelbelovende vormen van zorg en komt er inzicht in de effectiviteit en kosteneffectiviteit van deze zorg. Na afloop van de periode van voorwaardelijke toelating wordt besloten over daadwerkelijke toelating tot het verzekerde pakket.
Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
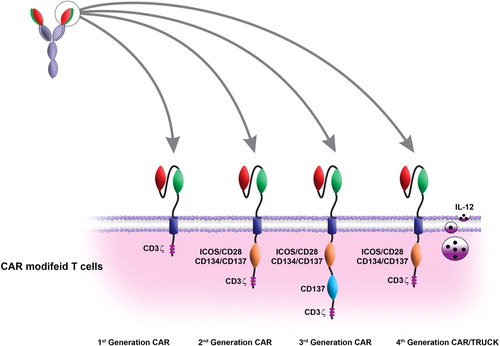
This review outlines four generations of CAR. The pre-clinical and clinical studies showed that this technique has a great potential for treatment of solid and hematological malignancies. The main purpose of the current review is to focus on the pre-clinical and clinical developments of CAR-based immunotherapy
CAR-modified T-cell therapy for cancer: an updated review
- DOI:
- 10.3109/21691401.2015.1052465
- Received: 17 Feb 2015
- Accepted: 24 Apr 2015
- Published online: 11 Jun 2015
Abstract
referentielijst van dendritische celtherapie met T-car cells - TIL - tumor Infilytrating Lymfocyten
References
- 1. Ahmed M, Cheung N-KV. 2013. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 588:288–297.
- 2. Beecham EJ, Ma Q, Ripley R, Junghans RP. 2000. Coupling CD28 co-stimulation to immunoglobulin T-cell receptor molecules: the dynamics of T-cell proliferation and death. J Immunother. 23:631–642.
- 3. Bollard CM, Rössig C, Calonge MJ, Huls MH, Wagner H-J, Massague J, . 2002. Adapting a transforming growth factor β–related tumor protection strategy to enhance antitumor immunity. Blood. 99:3179–3187.
- 4. Borkner L, Kaiser A, Van De Kasteele W, Andreesen R, Mackensen A, Haanen JB, . 2010. RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol Immunother. 59:1173–1183.
- 5. Brayer JB, Pinilla-Ibarz J. 2013. Developing strategies in the immunotherapy of leukemias. Cancer Control. 20:49–59.
- 6. Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, . 2011. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828. , , [Web of Science ®]
- 7. Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, . 2007. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 13:5426–5435. , , [Web of Science ®]
- 8. Bretscher PA. 1999. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA. 96: 185–190. , , [Web of Science ®]
- 9. Brocker T. 2000. Chimeric Fv-ζ or Fv-ε receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 96:1999–2001.
- 10. Burks A, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, . 2013. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 131:1288–1296.e3. , , [Web of Science ®]
- 11. Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, . 2009. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 106:3360–3365. , , [Web of Science ®]
- 12. Chambers CA, Allison JP. 1997. Co-stimulation in T cell responses. Curr Opin Immunol. 9:396–404.
- 13. Chang L, Chang W, Mcnamara G, Aguilar B, Ostberg J, Jensen M. 2007. Transgene-enforced co-stimulation of CD4+ T cells leads to enhanced and sustained anti-tumor effector functioning. Cytotherapy. 9:771–784. [Taylor & Francis Online], [Web of Science ®]
- 14. Chekmasova AA, Rao TD, Nikhamin Y, Park KJ, Levine DA, Spriggs DR, Brentjens RJ. 2010. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 16:3594–3606. , , [Web of Science ®]
- 15. Chen L. 2004. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol. 4:336–347. ,
- 16. Chen L, Flies DB. 2013. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 13:227–242. , , [Web of Science ®]
- 17. Chmielewski M, Kopecky C, Hombach AA, Abken H. 2011. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71:5697–5706. , , [Web of Science ®]
- 18. Chu J, Deng Y, Benson D, He S, Hughes T, Zhang J, . 2014. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 28:917–927. , , [Web of Science ®]
- 19. Croft M. 2005. The evolving crosstalk between co-stimulatory and co-inhibitory receptors: HVEM–BTLA. Trends Immunol. 26:292–294. ,
- 20. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, . 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 10:942–949. , , [Web of Science ®]
- 21. Darcy PK, Kershaw MH, Trapani JA, Smyth MJ. 1998. Expression in cytotoxic T lymphocytes of a single-chain anti-carcinoembryonic antigen antibody. Redirected Fas ligand-mediated lysis of colon carcinoma. Eur J Immunol. 28:1663–1672.
- 22. Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, . 2009. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 113:6392–6402. , , [Web of Science ®]
- 23. Durrant DM, Metzger DW. 2010. IL-12 can alleviate Th17-mediated allergic lung inflammation through induction of pulmonary IL-10 expression. Mucosal Immunol. 3:301–311. , , [Web of Science ®]
- 24. Eshhar Z, Waks T, Gross G, Schindler DG. 1993. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 90:720–724. , , [Web of Science ®]
- 25. Fenoglio D, Traverso P, Parodi A, Kalli F, Zanetti M, Filaci G. 2013. Generation of more effective cancer vaccines. Hum Vaccin Immunother. 9:2543–2547. [Taylor & Francis Online]
- 26. Finney HM, Akbar AN, Lawson AD. 2004. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCRζ chain. J Immunol. 172:104–113. ,
- 27. Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, . 2005. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8 + T cells. J Exp Med. 202:907–912. ,
- 28. Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, . 2014. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor–modified T cells. Blood. 123:2343–2354.
- 29. Goldberger O, Volovitz I, Machlenkin A, Vadai E, Tzehoval E, Eisenbach L. 2008. Exuberated numbers of tumor-specific T cells result in tumor escape. Cancer Res. 68:3450–3457.
- 30. Gong MC, Latouche J-B, Krause A, Heston WD, Bander NH, Sadelain M. 1999. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1:123. ,
- 31. Gorelik L, Flavell RA. 2002. Transforming growth factor-β in T-cell biology. Nat Rev Immunol. 2:46–53. ,
- 32. Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, . 2013. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2:e105. ,
- 33. Gross G, Waks T, Eshhar Z. 1989. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 86:10024–10028. , , [Web of Science ®]
- 34. Grossi JA, Raulet DH, Allison JP. 1992. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 356:607–609.
- 35. Haynes NM, Snook MB, Trapani JA, Cerruti L, Jane SM, Smyth MJ, Darcy PK. 2001. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-ζ vs FcεRI-γ. J Immunol. 166:182–187.
- 36. Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, . 2002. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol. 169:5780–5786. , , [Web of Science ®]
- 37. Heuser C, Hombach A, Lösch C, Manista K, Abken H. 2003. T-cell activation by recombinant immunoreceptors: impact of the intracellular signalling domain on the stability of receptor expression and antigen-specific activation of grafted T cells. Gene Ther. 10:1408–1419.
- 38. Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, . 2009. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 32:169. , , [Web of Science ®]
- 39. Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Kruis W, . 1997. T cell targeting of TAG72 + tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. Gastroenterology. 113:1163–1170.
- 40. Hombach A, Schneider C, Sent D, Koch D, Willemsen RA, Diehl V, . 2000. An entirely humanized CD3 zeta-chain signaling receptor that directs peripheral blood t cells to specific lysis of carcinoembryonic antigen-positive tumor cells. Int J Cancer. 88:115–120.
- 41. Hombach A, Sent D, Schneider C, Heuser C, Koch D, Pohl C, . 2001a. T-Cell activation by recombinant receptors cd28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res. 61:1976–1982.
- 42. Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, . 2001b. Tumor-specific T cell activation by recombinant immunoreceptors: CD3ζ signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J Immunol. 167:6123–6131. ,
- 43. Hosseini M, Haji-Fatahaliha M, Jadidi-Niaragh F, Majidi J, Yousefi M. 2015. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif Cells Nanomed Biotechnol. 1–11.
- 44. Hu Z, Xia J, Wargo J, Yang Y-G. 2013. Generation of human T cells expressing only the engineered tumor antigen specific TCR in humanized mice for preclinical research and anticancer therapy (P4364). J Immunol. 190:177.15.
- 45. Hudecek M, Lupo-Stanghellini M-T, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, Riddell SR. 2013. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 19:3153–3164. , , [Web of Science ®]
- 46. Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, . 2010. The B-cell tumor–associated antigen ROR1 can be targeted with T cells modified to express a ROR1- specific chimeric antigen receptor. Blood. 116:4532–4541. ,
- 47. Hwu P, Shafer G, Treisman J, Schindler D, Gross G, Cowherd R, . 1993. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 178:361–366. , , [Web of Science ®]
- 48. Jacobson CA, Ritz J. 2011. Time to put the CAR-T before the horse. Blood. 118:4761–4762.
- 49. Jameson SC, Masopust D. 2009. Diversity in T cell memory: an embarrassment of riches. Immunity. 31:859–871. , , [Web of Science ®]
- 50. Jena B, Maiti S, Huls H, Singh H, Lee DA, Champlin RE, Cooper LJ. 2013. Chimeric antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials. PloS one. 8:e57838.
- 51. Jensen MC, Popplewell L, Cooper LJ, Digiusto D, Kalos M, Ostberg JR, Forman SJ. 2010. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19- specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 16:1245–1256. ,
- 52. June CH. 2007. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 117:1466–1476. , , [Web of Science ®]
- 53. Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. 2004. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 64:9160–9166. , , [Web of Science ®]
- 54. Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, . 2012. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer. 106:1123–1133. ,
- 55. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. 2011. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 3:95ra73.
- 56. Katz SC, Burga RA, Mccormack E, Wang LJ, Mooring JW, Point G, . 2015. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor modified T cell therapy for CEA+ liver metastases. Clin Cancer Res. 1421.2014.
- 57. Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. 2015. Immunotherapeutic approaches for cancer therapy: an updated review. Artif Cells Nanomed Biotechnol. 1–11.
- 58. Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, . 2011. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 121:4746. , , [Web of Science ®]
- 59. Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, . 2010. Tumor-specific CD8 + T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 70:6725–6734. ,
- 60. Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, . 2002. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 13:1971–1980. , , [Web of Science ®]
- 61. Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, . 2006. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 12:6106–6115. , , [Web of Science ®]
- 62. Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, . 2012. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood. 119:2709–2720. ,
- 63. Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, . 2014. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 33:540–549.
- 64. Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, . 2009. Construction and Pre-clinical Evaluation of an Anti-CD19 Chimeric Antigen Receptor. J Immunother. 32:689.
- 65. Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler- Stevenson M, Feldman SA, . 2010. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 116: 4099–4102. , , [Web of Science ®]
- 66. Koehler H, Kofler D, Hombach A, Abken H. 2007. CD28 Costimulation Overcomes Transforming Growth Factor-β–Mediated Repression of Proliferation of Redirected Human CD4+ and CD8+ T Cells in an Antitumor Cell Attack. Cancer Res. 67:2265–2273.
- 67. Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. 2015. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 4:e994446. [Taylor & Francis Online]
- 68. Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, . 2006. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 66:10995–11004. , , [Web of Science ®]
- 69. Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, . 2012. TIL therapy broadens the tumor-reactive CD8 + T cell compartment in melanoma patients. Oncoimmunology. 1:409–418. [Taylor & Francis Online],
- 70. Ladygina N, Gottipati S, Ngo K, Castro G, Ma JY, Banie H, . 2013. PI3Kγ kinase activity is required for optimal T-cell activation and differentiation. Eur J Immunol. 43:3183–3196.
- 71. Lamers CH, Gratama JW, Warnaar SO, Stoter G, Bolhuis RL. 1995. Inhibition of bispecific monoclonal antibody (bsAb)-targeted cytolysis by human anti-mouse antibodies in ovarian carcinoma patients treated with bsAb-targeted activated T-lymphocytes. Int J Cancer. 60:450–457. , , [Web of Science ®]
- 72. Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, . 2006. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 24:e20–e22. ,
- 73. Lamers CH, Willemsen R, Van Elzakker P, Van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, . 2011. Immune responses to transgene and retroviral vector in patients treated with ex vivo–engineered T cells. Blood. 117:72–82.
- 74. Lee JC, Hayman E, Pegram HJ, Santos E, Heller G, Sadelain M, Brentjens R. 2011. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 71:2871–2881.
- 75. Lenschow DJ, Walunas TL, Bluestone JA. 1996. CD28/B7 system of T cell costimulation. Ann Rev Immunol. 14:233–258. , , [Web of Science ®]
- 76. Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner M. 2006. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 20:1819–1828. , , [Web of Science ®]
- 77. Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, . 2011. Antitumor activity and long-term fate of chimeric antigen receptor– positive T cells in patients with neuroblastoma. Blood. 118:6050–6056. ,
- 78. Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. 2002. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol. 20:70–75. ,
- 79. Mezzanzanica D, Canevari S, Mazzoni A, Figini M, Colnaghi MI, Waks T, . 1997. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 5:401–407.
- 80. Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, . 2013. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 36:133–151. , , [Web of Science ®]
- 81. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851. , , [Web of Science ®]
- 82. Muniappan A, Banapour B, Lebkowski J, Talib S. 2000. Ligand- mediated cytolysis of tumor cells: use of heregulin-ζ chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther. 7:128–134.
- 83. Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. 2006. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat Clin Pract Oncol. 3:668–681. ,
- 84. Murphy A, Westwood J, Brown L, Teng M, Moeller M, Xu Y, . 2007. Antitumor activity of dual-specific T cells and influenza virus. Cancer Gene Ther. 14:499–508. , , [Web of Science ®]
- 85. Näslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. 2013. Dendritic cell–derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 190:2712–2719. ,
- 86. Niederman TM, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC. 2002. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci USA. 99:7009–7014. , , [Web of Science ®]
- 87. Pameijer C, Navanjo A, Meechoovet B, Wagner J, Aguilar B, Wright C, . 2007. Conversion of a tumor-binding peptide identified by phage display to a functional chimeric T cell antigen receptor. Cancer Gene Ther. 14:91–97. , , [Web of Science ®]
- 88. Parente-Pereira AC, Burnet J, Ellison D, Foster J, Davies DM, Van Der Stegen S, . 2011. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J Clin Immunol. 31:710–718.
- 89. Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, . 2007. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 15:825–833. , [Web of Science ®]
- 90. Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. 2012. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 119:4133–4141. , , [Web of Science ®]
- 91. Perosa F, Favoino E, Caragnano MA, Prete M, Dammacco F. 2005. CD20: a target antigen for immunotherapy of autoimmune diseases. Autoimmun Rev. 4:526–531.
- 92. Pinthus JH, Waks T, Kaufman-Francis K, Schindler DG, Harmelin A, Kanety H, 2003. Immuno-gene therapy of established prostate tumors using chimeric receptor-redirected human lymphocytes. Cancer Res. 63:2470–2476. , [Web of Science ®]
- 93. Porter DL, Levine BL, Kalos M, Bagg A, June CH. 2011. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med. 365:725–733. , , [Web of Science ®]
- 94. Pranchevicius M-CS, Vieira TR. 2013. Production of recombinant immunotherapeutics for anticancer treatment: The role of bioengineering. Bioengineered. 4:305–312. [Taylor & Francis Online]
- 95. Pritchard M, Wolf S, Kraybill W, Repasky EA. 2005. The anti-tumor effect of interleukin-12 is enhanced by mild (fever-range) thermal therapy. Immunol Invest. 34:361–380. [Taylor & Francis Online], , [Web of Science ®]
- 96. Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, . 2008. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 14:1264–1270. , , [Web of Science ®]
- 97. Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. 2005. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 12:933–941. , , [Web of Science ®]
- 98. Rabinovich GA, Gabrilovich D, Sotomayor EM. 2007. Immunosuppressive strategies that are mediated by tumor cells. Ann Rev Immunol. 25:267. , , [Web of Science ®]
- 99. Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, . 2004. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 173:7125–7130. , , [Web of Science ®]
- 100. Rosenberg SA, Dudley ME. 2009. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 21:233–240. , , [Web of Science ®]
- 101. Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. 2002. Epstein-Barr virus–specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 99:2009–2016.
- 102. Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE. 2010. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther. 10:77–90. , , [Web of Science ®]
- 103. Sadelain M, Rivière I, Brentjens R. 2003. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 3:35–45. , , [Web of Science ®]
- 104. Santos EB, Yeh R, Lee J, Nikhamin Y, Punzalan B, Punzalan B, . 2009. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 15:338–344.
- 105. Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, . 2011. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J Clin Invest. 121:1822. ,
- 106. Schreiber RD, Old LJ, Smyth MJ. 2011. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 331:1565–1570. , , [Web of Science ®]
- 107. Seliger B. 2008. Different regulation of MHC class I antigen processing components in human tumors. J Immunotoxicol. 5:361–367. [Taylor & Francis Online], [Web of Science ®]
- 108. Sharifzadeh Z, Rahbarizadeh F, Shokrgozar MA, Ahmadvand D, Mahboudi F, Jamnani FR, Moghimi SM. 2013. Genetically engineered T cells bearing chimeric nanoconstructed receptors harboring TAG-72-specific camelid single domain antibodies as targeting agents. Cancer Lett. 334:237–244.
- 109. Smith-Garvin JE, Koretzky GA, Jordan MS. 2009. T cell activation. Ann Rev Immunol. 27:591–619. , , [Web of Science ®]
- 110. Spear P, Barber A, Rynda-Apple A, Sentman CL. 2012. Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-γ and GM-CSF. J Immunol. 188:6389–6398. , , [Web of Science ®]
- 111. Stewart-Jones G, Wadle A, Hombach A, Shenderov E, Held G, Fischer E, . 2009. Rational development of high-affinity T-cell receptor-like antibodies. Proc Natl Acad Sci USA. 106:5784–5788.
- 112. Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, . 2010. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 18:2006–2017. , , [Web of Science ®]
- 113. Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. 2012. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 18:6436–6445.
- 114. Tassev D, Cheng M, Cheung N-K. 2012. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 19:84–100. , , [Web of Science ®]
- 115. Teng MW, Kershaw MH, Moeller M, Smyth MJ, Darcy PK. 2004. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther. 15:699–708. , , [Web of Science ®]
- 116. Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, . 2008. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 112:2261–2271. , , [Web of Science ®]
- 117. Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, . 2012. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood. 119:3940–3950. ,
- 118. Tomihara K, Curiel TJ, Zhang B. 2013. Optimization of immunotherapy in elderly cancer patients. Critical Reviews™ in Oncogenesis. 18.
- 119. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mcdermott DF, . 2012. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 366:2443–2454. , , [Web of Science ®]
- 120. Trinchieri G, Pflanz S, Kastelein RA. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 19:641–644. , , [Web of Science ®]
- 121. Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, . 2012. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 72:1844–1852.
- 122. Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, . 2006. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 108:3890–3897. , , [Web of Science ®]
- 123. Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, . 2007. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 18:712–725. , , [Web of Science ®]
- 124. Wang J, Press OW, Lindgren CG, Greenberg P, Riddell S, Qian X, . 2004. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8 + cytotoxic T lymphocytes. Mol Ther. 9:577–586.
- 125. Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, . 2005. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci USA. 102:19051–19056. , , [Web of Science ®]
- 126. Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, . 2011. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 19:751–759. , , [Web of Science ®]
- 127. Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, . 2009. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 183:5563–5574. , , [Web of Science ®]
- 128. Zhong X-S, Matsushita M, Plotkin J, Riviere I, Sadelain M. 2010. Chimeric Antigen Receptors Combining 4–1BB and CD28 Signaling Domains Augment PI3kinase/AKT/Bcl-XL Activation and CD8 & plus; T Cell–mediated Tumor Eradication. Mol Ther. 18:413–420. ,
- 129. Zou Y, Stastny P, Süsal C, Döhler B, Opelz G. 2007. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 357: 1293–1300. , , [Web of Science ®]
Gerelateerde artikelen
- Wat houdt CAR-T celtherapie in? Zie overzicht en stand van zaken. Met extra aandacht voor CAR-T celt therapie bij Acute Myeloide Leukemie
- CAR-T celtherapie gericht op de CLL1 en CD33 expressie met dubbele aanpak geeft hoopvolle resultaten bij Acute Myeloide Leukemie. Een vrouw met recidief van AML na beenmergtransplantatie komt zelfs in een duurzame complete remissie
- CAR-T-celtherapie geneest patienten met Lupus en leukemie maar is duur, ziekmakende afweercellen wegzuigen uit bloed van proefdieren via mRNA is even effectief en goedkoper
- CAR-T celtherapie maakt dat kinderen met neuroblastoma nu in 2025 al meer dan 10 jaar kankervrij zijn. Met uitgelicht een prachtig overlevingsverhaal van een kind van 4 jaar die anno 2025 al 19 jaar kankervrij is.
- CD19 Fast-CAR-T-cel therapie bij patienten met vergevorderde B-cel acute lymfatische leukemie (B-ALL) geven hele goede resultaten bij 15 van de 20 patienten langdurige complete remissies
- CAR-T celtherapie brengt binnen 1 jaar al 7 van de 44 patienten met leukemie en lymfklierkanker in totale remissie in Spanje in een open gezondheidsprogramma
- CAR-T celtherapie is zeer succesvol bij kankerpatienten maar loopt in Nederland vast op te strenge milieueisen, stellen Nederlandse top wetenschappers copy 1
- Immuuntherapie met gemanipuleerde T-cellen geeft spectaculair goede resultaten bij patienten met vergevorderde leukemie en B-cel lymfomen
- Immuuntherapie met gemanipuleerde T-cellen - CAR-T celtherapie ( tisagenlecleucel ) geeft spectaculair goede resultaten bij patienten met gevorderde lymfklierkanker - non-Hodgkin (B-lymfomen)
- Dendritische celtherapie met T-car cells wordt als proef vergoed vanuit basiszorgverzekering voor melanomen stadium IIIB en IIIC
- Longkanker: Dendritische celtherapie plus gemoduleerde T-cellen naast chemo geeft 25 procent betere mediane overall overleving op 5 jaar bij operabele niet-kleincellige longkanker
- Immuuntherapie met TIL - tumor infiltrating lymfocyten zorgt bij een kwart van de deelnemers voor jarenlange ziektevrije tijd bij patienten met uitgezaaide melanomen
- Immuuntherapie met CAR T-Cell Therapy (tisagenlecleucel (Kymriah) goedgekeurd door FDA voor gebruik bij kinderen en jong volwassenen met vorm van Acute Lymfatische Leukemie (ALL). copy 1 copy 1
- Immuuntherapie met T-car cells - Tumor Lymphocytic Infiltration is interessante ontwikkeling bij verschillende vormen van kanker. Hoe meer TLI hoe beter
- Immuuntherapie met gemoduleerde T-car cells geeft bij zwaar voorbehandelde lymfklierkanker non-Hodgkin alsnog uitstekende resultaten met 33 en 50 procent complete remissies
- Receptor-gemodificeerde T-cellen leiden tot totale remissies bij uitbehandelde agressieve acute lymfatische leukemie - ALL en geven hoop op genezende behandeling. copy 1
- Autologe genetisch gemodificeerde T-cellen gericht tegen het humaan papillomavirus (HPV) 16 E6 geeft bij patienten met vergevorderde zwaar voorbehandelde uitgezaaide HPV gerelateerde kanker uitstekende resultaten
- CAR-T cel therapie is een vorm van immuuntherapie die hele goede resultaten geeft. In Jama een overzicht van stand van zaken in de klinische praktijk



Plaats een reactie ...
Reageer op "Dendritische celtherapie met T-car cells wordt als proef vergoed vanuit basiszorgverzekering voor melanomen stadium IIIB en IIIC"