Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven waaronder bij MEDpro op prostasol of prostectan voor prostaatkanker welke goed te combineren is met granaatappelsap:
2 november 2015:
Granaatappelsap aanvullend op eerstelijnsbehandeling verlengt bij prostaatkankerpatiënten met het MnSOD AA genotype het tijdstip tot zich een PSA stijging voordoet met gemiddeld een jaar 13,6 tot 25,6 maanden, in vergelijking met een placebo naast een standaard behandeling. Het verschil tussen een placebo en een extract van granaatappelsap was minder en niet statistisch significant, maar wel nog beter. Bij alle deelnemende patiënten werd er wel een verschil gevonden van enkele maanden maar dit was niet statistisch significant. Interessant overigens dat zowel een placebo als een extract een positief effect had op de snelheid van de PSA stijging. Maar het granaatsap zelf gaf de beste resultaten bij vooral patiënten (bij 22% werd dit type gevonden uit alle deelnemende patiënten) met het MnSOD AA genotype.
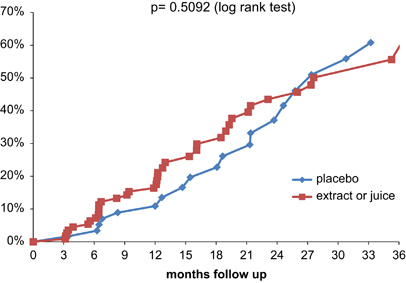
Dit is een gedegen opgezette studie zover ik dat als leek kan beoordelen. Maar oordeelt u zelf maar, het volledige studierapport: A randomized, double-blind, placebo-controlled study of the effects of pomegranate extract on rising PSA levels in men following primary therapy for prostate cancer is gratis in te zien. Zie ook toegevoegde referentielijst.
Het voilledige studierapport van een eerdere fase II studie, zie hieronder artikel daarover: Phase II Study of Pomegranate Juice for Men with Rising Prostate-Specific Antigen following Surgery or Radiation for Prostate Cancer is ook gratis in te zien en toont de waarde van granaatappelsap om PSA stijging te remmen.
Het abstract van de recente studie staat onderaan artikel. Hieronder grafiek van deelnemende patienten uit deze studie verdeeld over de groepen.
2 juni: bron: Reuters
8 ons granaatappelsap = ca. twee glazen per dag - lijkt een sterk remmend effect te hebben op prostaatkankergroei geassocieerd met PSA stijging na operatie of bestraling van de prostaatkanker. Op ASCO 2005 presenteerden onderzoekers een fase II studie waarbij 48 mannen met recideif van prostaatkanker zijn gevolgd die of een operatie hadden gehad of waren bestraald voor hun prostaatkanker na stijgende PSA waarden. Na de behandeling kregen de mannen elke dag 8 ounces = ca. 225 ml. is een longdrinkglas granaatappelsap te drinken en niets anders. Ten opzichte van statistische gegevens waarin een gemiddelde tijd tot nieuwe PSA stijging ligt op 15 maanden tot nieuwe PSA stijging werd nu deze tijd met het granaatappelsap verlengt tot maar liefst 37 maanden, een winst dus van bijna twee jaar. De onderzoekers gaan nu een fase III studie hiermee opzetten. Het is al langer bekend dat granaatappels veel anti-oxidanten bevatten, maar lijkt nu ook effect te hebben door de phyto-oestrogene capaciteiten. Met dank aan Peter die ons vertelde dat bij sommige Turkse winkels dit granaatsap is te verkrijgen. Er zit wel suiker in, maar zonder suiker lijkt het niet te drinken zo zuur, althans dat vertelde Peter ons. Met dank aan Gert die ons het abstract stuurde van de studie en daaronder artikel van Reuters
Publishing #: 831
Presentation Title: Phase II Study of Pomegranate Juice for Men with Rising PSA following Surgery or Radiation for Prostate Cancer
Category: 44 Advanced
Author Block: Allan J Pantuck*, John T. Leppert, Nazy Zomorodian, Navindra Seeram, Daniel Seiler, Harley Liker, He-jing Wang, Robert Elashoff, David Heber, Arie Belldegrun, Los Angeles, CA
Introduction and Objective: Phytochemicals in edible plants can have cancer preventive benefits through antioxidation and via gene-nutrient interactions. Pomegranate juice has been shown to be a rich source of polyphenolic flavonoids. Pre-clinical data suggested the ability of pomegranate juice to modulate the growth and progression of prostate cancer. To determine the clinical effects of pomegranate juice on patients with prostate cancer, a clinical trial was performed. Methods: A 2 year, single center, phase II, Simon two stage clinical trial for men with rising PSA after surgery or radiotherapy was designed based on a 20% response rate, an alpha of 5%, and 90% power. Eligible patients had a detectable PSA greater than 0.2 ng/ml and less than 5 ng/ml, and a Gleason score of 7 or less. Serial PSA measurements determined a baseline PSA doubling time. Patients were treated with 8 ounces of pomegranate juice by mouth daily (wonderful variety, equivalent to 1.5 mmol of total polyphenols per day) until disease progression. Clinical endpoints included safety, effect on serum PSA, and exploratory laboratory studies. Patients were followed in 3 month intervals for serum PSA, and blood and urine were collected for laboratory studies. Results: The study was fully accrued to 48 participants in two stages after efficacy criteria were met. There were no serious adverse events reported and the treatment was well tolerated. No patients developed metatsatic disease on study. Mean PSA doubling time significantly increased with treatment, from a mean of 14 to 26 months (p< 0.048). The slope of the mean log PSA decreased from 0.08 to 0.04 on treatment (p< 0.019). In vitro assays using pre and post treatment patient serum on the growth of LNCaP showed decreased cell proliferation and increased apoptosis (p<0.07). Pomegranate polyphenols were detected in the urine of all participants by LC-MS. Conclusions: We report the first clinical trial of pomegranate juice in patients with recurrent prostate cancer. The positive and significant beneficial effects on PSA parameters achieved, coupled with corresponding laboratory effects on prostate cancer in vitro cell growth and apoptosis warrent further testing in a randomized, placebo controlled phase III study. Keywords: Prostate cancer,Antineoplastic agents,Antioxidants
,br> American Urological Association Annual Meeting
May 21 - 26, 2005
San Antonio, Texas, USA
Monday, May 23, 2005
NEW YORK (Reuters Health) - In men with recurrent prostate cancer, drinking 8 ounces per day of pomegranate juice significantly increases the time it takes for prostate specific antigen (PSA) levels to rise, according to research presented Monday at the annual meeting of the American Urological Association in San Antonio.
Before the men in the study began consuming pomegranate juice, the average PSA doubling time, a measure of tumor activity, was 15 months, lead author Dr. Allan J. Pantuck, from the University of California at Los Angeles, told Reuters Health. "The average time after treatment was 37 months. So, there was almost a 2-year increase in the doubling time."
Pomegranate juice contains a number of antioxidants thought to have anti-cancer effects, Pantuck noted. In addition, the juice contains estrogen-like plant substances called phytoestrogens that could be useful in combatting prostate cancer, in particular.
Based on encouraging results in cell culture and in animal models of prostate cancer, Pantuck said his team decided to conduct a clinical trial of pomegranate juice in 48 men with rising PSA levels after surgery or radiation therapy for their malignancy. As noted, the subjects were instructed to consume 8 ounces of pomegranate juice daily.
Pomegranate juice therapy was well tolerated and no serious adverse effects were reported. In addition to the beneficial increase in PSA doubling time, in vitro testing showed decreased cancer cell division and proliferation and increased cancer cell death. Urine testing confirmed the presence of pomegranate antioxidants in all men.
Given these promising findings, Pantuck noted that a multicenter phase III randomized trial is now in the works.
The study was funded by the Stewart and Lynda Resnick Trust, which own the POM Wonderful pomegranate juice company.
A randomized, double-blind, placebo-controlled study of the effects of pomegranate extract on rising PSA levels in men following primary therapy for prostate cancer
A randomized, double-blind, placebo-controlled study of the effects of pomegranate extract on rising PSA levels in men following primary therapy for prostate cancer.
Abstract
BACKGROUND:
The primary objective of this study was to compare the effects of pomegranate juice on PSA doubling times (PSADT) in subjects with rising PSA levels after primary therapy for prostate cancer.
METHODS:
Double-blind, placebo-controlled multi-institutional study, evaluated the effects of pomegranate liquid extract on serum PSA levels. The primary end point of this study was change in serum PSADT. Additional secondary and exploratory objectives were to evaluate the safety of pomegranate juice and to determine the interaction of manganese superoxide dismutase (MnSOD) AA genotype and pomegranate treatment on PSADT.
RESULTS:
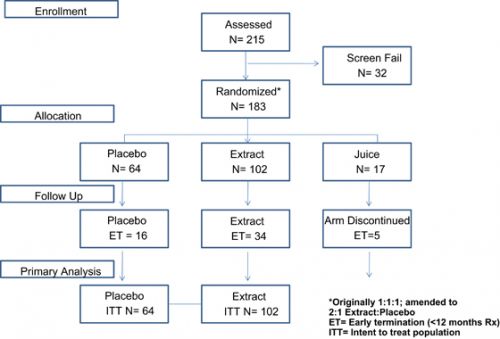
One-hundred eighty-three eligible subjects were randomly assigned to the active and placebo groups with a ratio of 2:1 (extract N=102; placebo N=64; juice N=17). The majority of adverse events were of moderate or mild grade. Median PSADT increased from 11.1 months at baseline to 15.6 months in the placebo group (P<0.001) compared with an increase from 12.9 months at baseline to 14.5 months in the extract group (P=0.13) and an increase from 12.7 at baseline to 20.3 in the juice group (P=0.004). However, none of these changes were statistically significant between the three groups (P>0.05). Placebo AA patients experienced a 1.8 month change in median PSADT from 10.9 months at baseline to 12.7 months (P=0.22), while extract patients experienced a 12 month change in median PSADT from 13.6 at baseline to 25.6 months (P=0.03).
CONCLUSIONS:
Compared with placebo, pomegranate extract did not significantly prolong PSADT in prostate cancer patients with rising PSA after primary therapy. A significant prolongation in PSADT was observed in both the treatment and placebo arms. Men with the MnSOD AA genotype may represent a group that is more sensitive to the antiproliferative effects of pomegranate on PSADT; however, this finding requires prospective hypothesis testing and validation.
- PMID:
- 26169045
- [PubMed - in process]
-
References
- Bishop FL, Rea A, Lewith H, Chan YK, Saville J, Prescott P et al. Complementary medicine use by men with prostate cancer: a systematic review of prevalence studies. Prostate Cancer Prostatic Dis 2011; 14: 1–13. | Article | PubMed |
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 2000; 71: 1062–1076. | PubMed | ISI | CAS |
- Rettig MB, Heber D, An J, Seeram NP, Rao JY, Liu H et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol Cancer Ther 2008; 7: 2662–2671. | Article | PubMed | ISI | CAS |
- Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem 2007; 55: 7732–7737. | Article | PubMed |
- Koyama S, Cobb LJ, Mehta HH, Seeram NP, Heber D, Pantuck AJ et al. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm IGF Res 2010; 20: 55–62. | Article | PubMed |
- Sartippour MR, Seeram NP, Rao JY, Moro A, Harris DM, Henning SM et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int J Oncol 2008; 32: 475–480. | PubMed | ISI | CAS |
- Rettig MB, Heber D, An J, Seeram NP, Rao JY, Liu H et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol Cancer Ther 2008; 7: 2662–2671. | Article | PubMed | ISI | CAS |
- Adhami VM, Siddiqui IA, Syed DN, Lall RK, Mukhtar H. Oral infusion of pomegranate fruit extract inhibits prostate carcinogenesis in the TRAMP model. Carcinogenesis 2012; 33: 644–651. | Article | PubMed |
- Wang L, Alcon A, Yuan H, Ho J, Li QJ, Martins-Green M. Cellular and molecular mechanisms of pomegranate juice-induced anti-metastatic effect on prostate cancer cells. Integr Biol (Camb) 2011; 3: 742–754. | Article | PubMed |
- Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res 2006; 12: 4018–4026. | Article | PubMed | ISI | CAS |
- Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis 2012; 16: 50–55. | Article | PubMed |
- Smith MR, Manola J, Kaufman DS, George D, Oh WK, Mueller E et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer 2004; 101: 1569–1574. | Article | PubMed | ISI |
- Li H, Kantoff P, Giovannucci E, Leitzmann MF, Gaziano JM, Stampfer MJ et al. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinically significant prostate cancer risk. Cancer Res 2005; 65: 2498–2504. | Article | PubMed | ISI | CAS |
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. | Article | PubMed | ISI |
- Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. J Urol 2007; 178: S20–S24. | Article | PubMed | ISI |
- Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007; 51: 1175–1184. | Article | PubMed | ISI |
- Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res 1992; 52: 2071s–2077s. | PubMed | CAS |
- Malins DC, Johnson PM, Barker EA, Polissar NL, Wheeler TM, Anderson KM. Cancer-related changes in prostate DNA as men age and early identification of metastasis in primary prostate tumors. Proc Natl Acad Sci USA 2003; 100: 5401–5406. | Article | PubMed | CAS |
- Gurel B, Lucia MS, Thompson IM, Goodman PJ, Tangen CM, Kristal AR et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Can Epi Biom Prevention 2014; 23: 847–856. | Article |
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007; 7: 256–269. | Article | PubMed | ISI | CAS |
- Trzeciak AR, Nyaga SG, Jaruga P, Lohani A, Dizdaroglu M, Evans MK. Cellular repair of oxidatively induced DNA base lesions is defective in prostate cancer cell lines, PC-3 and DU-145. Carcinogenesis 2004; 25: 1359–1370. | Article | PubMed | ISI | CAS |
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1988; 56: 317–333. | Article |
- Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food 2004; 7: 274–283. | Article | PubMed |
- Gasmi J, Sanderson JT. Growth inhibitory, antiandrogenic, and pro-apoptotic effects of punicic acid in lncap human prostate cancer cells. J Agric Food Chem 2010; 58: 12149–12156. | Article | PubMed |
- Koyama S, Cobb LJ, Mehta HH, Seeram NP, Heber D, Pantuck AJ et al. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm IGF Res 2010; 20: 55–62. | Article | PubMed |
- Wang L, Alcon A, Yuan H, Ho J, Li QJ, Martins-Green M. Cellular and molecular mechanisms of pomegranate juice-induced anti-metastatic effect on prostate cancer cells. Integr Biol (Camb) 2011; 3: 742–754. | Article | PubMed |
- Domingo-Domenech J, Mellado B, Ferrer B, Truan D, Codony-Servat J, Sauleda S et al. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer 2005; 93: 1285–1294. | Article | PubMed | CAS |
- Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res 2004; 10: 8460–8464. | Article | PubMed | ISI | CAS |
- Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C et al. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate 2002; 52: 183–200. | Article | PubMed | ISI | CAS |
- Li N, Oberley RD, Oberley LW, Zhong W. Overexpression of manganese superoxide dismutase in DU145 human prostate cancer cells has multiple effects on cell phenotype. Prostate 1998; 35: 221–233. | Article | PubMed | ISI | CAS |
- Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics 2003; 13: 145–157. | Article | PubMed | CAS |
- Hong YC, Lee KH, Yi CH, Ha EH, Christiani DC. Genetic susceptibility of term pregnant women to oxidative damage. Toxicol Let 2002; 129: 255–262. | Article |
- Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Wu K, Erdman JW Jr et al. Manganese superoxide dismutase (MnSOD) gene polymorphism, interactions with carotenoid levels and prostate cancer risk. Carcinogenesis 2008; 29: 2335–2340. | Article | PubMed |
- D'Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 2003; 95: 1376–1383. | Article | PubMed |
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC et al. Risk of prostate cancer–specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–439. | Article | PubMed | ISI | CAS |
- Scher HI, Helabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Onc 2008; 26: 1148–1159. | Article |
- Paller CJ, Olatoye D, Xie S, Zhou X, Denmeade SR, Eisenberger MA et al. The effect of the frequency and duration of PSA measurement on PSA doubling time calculations in men with biochemically recurrent prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 28–33. | Article | PubMed |
- Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis-free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer 2012; 118: 1533–1542. | Article | PubMed | ISI | CAS |
Top of pageAcknowledgements
Study funding, pomegranate products and placebos provided by Pom Wonderful. Manuscript co-author AJP has received research funding from and DH has received research funding and is a paid consultant to Pom Wonderful. Study Principal Investigators include: RD (Taussig Cancer Center Cleveland Clinic), AK (Winthrop Medical Center), AJP (UCLA Institute of Urologic Oncology), JC (Virginia Mason Medical Center), CAP (University of Texas MD Anderson Cancer Center), Sheldon Freedman, Gary Karlin (AdvanceMed Research), WA (UCLA, West LA VAMC), Giribala Patel (St Jude Heritage Medical Group), Andre Liem (Pacific Shores Medical Group), Frederic Kass (Santa Barbara Hematology Oncology Group), Robert Dichman (Central Coast Medical Oncology Corporation), Brad Bauer (Alabama Clinical Research Institute), WC (Alaska Clinical Research Center), GS (Five Valleys Urology), Ron Israeli (Staten Island Urologic Research), Joseph Kuntze (Coastal Medical Research Group), Robert Kratzke (University of Minnesota). Contract Research Organization (CRO) clinical trial support services provided by Radiant Clinical Research, Chicago, IL, USA. Data management and statistical analysis performed by Daniela Markovic and Jeffrey Gornbein, PhD, from the UCLA Department of Biomathematics.
Gerelateerde artikelen
- Antioxidanten: Een mix van voedingsupplementen soja, isoflavones, lycopeen, silymarin en antioxidanten - vermindert de PSA stijging significant bij prostaatkankerpatiënten na operatieve ingreep of bestraling als postoperatieve behandeling
- Capsaicin is een stofje dat veel voorkomt in rode pepers bevordert apoptosis - zelfdoding van prostaatkankercellen.
- Cranberry extract verlaagt PSA met 22,5 procent binnen een maand bij prostaatkankerpatienten die wachten op operatie blijkt uit placebo gecontroleerde studie
- Curcumine: Een half jaar dagelijks curcumine (1440 mg/dag) stabiliseert PSA progressie in vergelijking met placebo veel beter (30 vs 10 procent) bij prostaatkankerpatiënten in hormoonvrije periode copy 1
- Ellagic-acid (Quercetin-C), veel voorkomend in bessen, rode vruchten en noten geeft significant minder bijwerkingen en significant betere respons wanneer aanvullend gegeven bij chemokuren voor recidief van prostaatkanker
- Granaatappelsap onderdrukt PSA stijging bij prostaatkanker van 15 maanden naar 37 maanden dus verlenging van PSA stabiliteit met circa twee jaar
- Groene thee extract heeft uitstekend effect bij prostaatkankerpatienten in een wait-and-see programma
- Groene thee bewijst opnieuw therapeutische waarde voor prostaatkanker - 27 procent verschil in ontwikkelen van prostaatkanker tussen placebo en groene thee groepgebruik
- Knoflook, uien en sjalotten lijken de kans op het krijgen van prostaatkanker met 50% te verminderen, aldus studie NCI.
- Koffie geeft langere prostaatkanker-specifieke overleving voor specifieke subgroepen, waaronder die met het CYP1A2 AA-genotype
- Leefstijl: Drastische verandering van leefstijl en overstappen op vegetarische voeding geeft significant betere resultaten bij prostaatkankerpatiënten, die in eerste instantie reguliere aanpak weigerden
- Lezing van Dean Ornish over de combinatie van voeding, supplementen, liefde en intimiteit die leidt tot een betere gezondheid en wellicht preventief en genezend kan zijn bij kanker copy 1
- Lijnzaadolie - flax seed oil zou prostaatkanker juist stimuleren beweert dr. William P. Marley. Visolie is veel beter dan lijnzaadolie, aldus Marley
- Lycopeen, een uitstekend middel om prostaatkanker te voorkomen en progressie te remmen. En lycopeen voorkomt hart- en vaatziektes. Een wetenschappelijke analyse van arts-bioloog drs. Engelbert Valstar
- Metformin en Zyflamend, een kruidencombinatie vergelijkbaar met prostasol stopt progressie van prostaatkanker waar hormoontherapie en chemo faalden bij vier mannen met vergevorderde prostaatkanker.
- Overzichtsstudies met effecten van leefstijl, voeding en voedingstoffen als preventie van of aanvullend bij prostaatkanker en reguliere behandelingen waaronder hormoontherapie
- Plantaardig dieet vermindert kans op recidief, verbetert overleving en verbetert kwaliteit van leven door betere sexualiteit en beter plassen bij prostaatkankerpatienten
- Pomi-T, een voedingssupplement dat granaatappel, broccoli, groene thee en kurkuma bevat verlaagt PSA waarden hoog significant - 14,7 vs 78,5 procent, P = 0,0008 - in vergelijking met placebo en vermindert significant tweede reguliere behandelingen.
- Prostaatkanker patienten met een verhoogde PSA hebben baat bij verandering van levensstijl en voedingspatroon blijkt uit gerandomiseerde studie. Niemand uit groep mannen die leefstijl veranderde had later behandeling nodig. Artikel geplaatst 16 juli 2010
- Resveratrol: Elke dag glas rode wijn - resveratrol bevattend - voorkomt het ontwikkelen van prostaatkanker.
- Selenium speelt wellicht een rol in uitzaaiingsproces bij prostaatkanker blijkt uit onderzoek aan Universiteit van Wageningen.
- Studie naar effect van vitamine E in combinatie met lycopeen bij prostaatkanker in Erasmus Medisch Centrum al gestart in oktober 2004
- Vetarm dieet met vlaszaad vermindert kans op krijgen van prostaatkanker
- Vetarmdieet van Dean Ornish zorgt voor stabilisatie en zelfs teruggang van kanker bij patiënten met prostaatkanker
- Vitamine D. waarden in bloed van oudere mannen geven risico aan op krijgen van prostaatkanker. hoe lager de waarden hoe groter het risico - dubbele voor laagste waarden - bij bepaalde agressieve vormen van prostaatkanker.
- Vitamine D als aanvulling op Docetaxel - Taxotere - zorgt voor sterke vermindering van PSA-waarde bij patiënten met vergevorderde prostaatkanker.
- Vitamine D en zonlicht heeft therapeutisch en preventief effect bij prostaatkanker blijkt uit grote overzichtstudie van gerandomiseerde studies Artikel geplaatst 28 april 2009
- Vitamine E: hoge vitamine E waarden in het bloed vermindert kans op krijgen van prostaatkanker met ca. 50 procent, aldus de conclusie uit de ATBC - Alpha-Tocopherol, Beta-Carotene Cancer Prevention studie
- Selenium kan kans op prostaatkanker met meer dan 30 procent verminderen en selenium lijkt daarmee ook belangrijk in het voorkomen van een recidief of remming van primaire prostaatkanker.
- Soja isoflavonen aanvullend op bestraling bij prostaatkanker verminderen bijwerkingen en zorgen voor minder impotentie.
- Sulfurofaan vertraagt stijging van PSA tot verdubbeling met 86 procent - 28.9 vs 15.5 maanden - bij prostaatkankerpatienten na operatie in vergelijking met placebo copy 1
- Vet arm dieet plus vlaszaad heeft aantoonbaar therapeutische effect bij prostaatkanker.
- Visolie: Omega-3 vetzuren - vette vis (LC n-3) voorkomt significant prostaatkanker en vermindert significant de agressiviteit van prostaatkanker als die eenmaal is geconstateerd. Blijkt uit vergelijkende studie met 900 mannen
- Voedingsinterventie bij prostaatkankerpatienten geeft twee tegengestelde uitslagen uit twee gerandomiseerde Nederlandse studies.
- Wait and see beleid aangevuld met voeding en leefstijladviezen bij prostaatkankerpatienten voorkomt bij 80 tot 100 procent dat er nog een behandeling moet volgen.
- Voeding en voedingstoffen bij prostaatkanker. Een aantal artikelen en studies over effecten van voeding en voedingstoffen als preventie of therapeutisch bij prostaatkanker bij elkaar gezet. Update 22 juni 2010



Plaats een reactie ...
1 Reactie op "Granaatappelsap onderdrukt PSA stijging bij prostaatkanker van 15 maanden naar 37 maanden dus verlenging van PSA stabiliteit met circa twee jaar"