8 februari 2017: Bron: ASH 2016 San Diego.
Wanneer zwaar voorbehandelde patienten met vergevorderde botkanker - multiple myeloma (Kahler) een combinatie behandeling krijgen van Nelfinavir plus bortezomib en dexamethasone dan bereikte 65% van de deelnemende patienten (22 uit 34 patienten) alnsog een gedeelteljike remissie van 50 procent of meer. De algemene kliniche respons, waarin ook stabiele ziekte en minimale remissies werden meegenomen was maar liefst 86 procent.
Tijdens de ASH werden dit de kernpunten genoemd van deze studie:
Nelfinavir in Multiple Myeloma
- Het antiretrovirale medicijn nelfinavir toont sterke klinische activiteit bij zwaarvoorbehandelde patienten met resistente teruggekeerde multiple myeloma (Kahler) volgens een kleine fase II studie met 34 patienten.
- De response was 65%, inclusief een 15% zeer goede gedeelteljike remissie, van 50% of meer. Bij patiënten met een hoog risico met zogenaamde "cytogenetics", was de response 77%.
- De verdere ontwikkeling van dit medicijn met veel potentie is echter onzeker omdat het patent op nelfinavir afloopt.
Bron: https://ash.confex.com/ash/2016/webprogram/Paper89818.html
Studieresultaten:
Positieve klinische reacties werd gezien bij 22 patiënten uit 34 (= 65%) die de nelfinavir plus behandeling kregen. Er werden zeer goede remissies gezien (tussen de 50 en 100 procent) bij 5 patienten (15%) en gedeeltelijke remissies (een PR van gelijk of meer dan 50%) bij 17 patiënten (50%). 3 patiënten (9%) had een remissie kleiner dan 50% en 4 anderen (12%) hadden stabiele ziekte, dus stopte de progressie van de ziekte, resulterend in een klinische respons van 86%. Klinische effectiviteit werd algemeen gezien bij alle patienten ongeacht de verschillende voorbehandelingen van bv. stamceltransplantatie of chemo of andere behandelingen.
Ik kan niet zo heel veel meer vertellen over deze studie: The HIV Protease Inhibitor Nelfinavir in Combination with Bortezomib and Dexamethasone (NVd) Has Excellent Activity in Patients with Advanced, Proteasome Inhibitor-Refractory Multiple Myeloma: A Multicenter Phase II Trial (SAKK 39/13)
behalve dan het abstract zoals dat op de ASH werd gepresenteerd. Maar bedenk dat alle patienten minimaal 5 andere behandeliingen eerder hebben gehad en dan toch nog zo goed reageerden op deze behandeling is natuurlijk heel goed nieuws.
Nelfinavir in combination with bortezomib and dexamethasone (NVd) is a reasonable, active, safe and widely available treatment option for patients with proteasome inhibitor-refractory multiple myeloma. The objective response rate of 65% observed in this very advanced, heavily pretreated, mostly dual-refractory patient population is exceptional. Our results warrant further development of nelfinavir as a sensitizing drug for proteasome inhibitor-based treatments and promising new agent for MM therapy.
The HIV Protease Inhibitor Nelfinavir in Combination with Bortezomib and Dexamethasone (NVd) Has Excellent Activity in Patients with Advanced, Proteasome Inhibitor-Refractory Multiple Myeloma: A Multicenter Phase II Trial (SAKK 39/13)
Program: Oral and Poster Abstracts
Type: Oral
Session: 653. Myeloma: Therapy, excluding Transplantation: New Agents for Multiple Myeloma
Rationale: Proteasome inhibitor-refractory multiple myeloma (MM) patients are a difficult to treat population with a very poor prognosis. The activity of registered, current or next generation MM drugs (pomalidomide, carfilzomib, daratumumab) in heavily pretreated, proteasome inhibitor-refractory MM is in the range of 30%. The biology of proteasome inhibitor resistance is driven by adaptive downregulation of the unfolded protein response (UPR), which regulates plasma cell maturation and sensitivity to proteasome inhibitor treatment (Leung-Hagesteijn C. et al., Cancer Cell 2013 Sep 9;24(3):289-304). The oral HIV protease inhibitor nelfinavir (NFV) has anti-MM activity in vivo, triggers UPR activation, sensitizes MM to proteasome inhibitors and overcomes proteasome inhibitor resistance in vitro. Combination therapy with NFV and bortezomib (BTZ) showed UPR activation in vivo and strong signals of activity in bortezomib-refractory MM in the SAKK 65/08 phase I trial (Driessen C. et al., Haematologica 2016 Mar;101(3):346-55).
Objective: We performed a prospective, multicenter phase II trial to assess the activity of nelfinavir, bortezomib and dexamethasone (NVd) in proteasome inhibitor-refractory MM.
Methods: Patients with progressing, measurable, proteasome inhibitor-refractory MM (IMWG criteria) were included in this multicenter phase II trial. Further selection criteria included WHO performance status ≤ 3, platelets ≥ 50 x 109/L, hemoglobin ≥ 80 g/L (both may be achieved by transfusion) and adequate hepatic function. Concomitant use of other anti-cancer medication or radiotherapy, except for local pain control, was excluded. Patient age or prior number or types of therapy were not limited. Simon’s two stage design was used to differentiate a promising activity (best response at any time point, partial response (PR) or better, ≥ 35 %) from an uninteresting activity (≤ 15% PR; power 80%, alpha 5%).
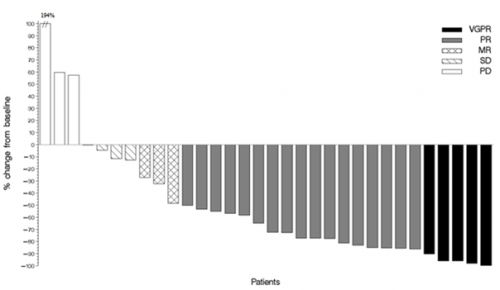
Results: 34 patients were treated with oral nelfinavir 2500 mg days 1-14 b.i.d. in combination with bortezomib + dexamethasone (BTZ 1.3 mg/m2 days 1, 4, 8, 11, dexamethasone 20 mg p.o. days 1-2, 4-5, 8-9, 11-12) for a maximum of 6 21-day cycles at 9 SAKK trial sites throughout Switzerland. Patients (median age 67.5 years, range 42-82 years) had a median of 5 (range 2-12) prior therapy lines, 26 (76%) patients had prior high dose chemotherapy, and 13 (39%) had known poor prognosis cytogenetic abnormalities. All treated patients had proteasome inhibitor refractory MM according to IMWG criteria, i.e. they had progressed during or within 60 days after adequately dosed proteasome inhibitor-containing therapy. Moreover, 26 (76%) patients were lenalidomide-refractory by IMWG criteria (double refractory). Trial therapy is still ongoing in 2 patients. The median number of treatment cycles delivered per patient is 4. 22 patients achieved an objective response with a PR or better, resulting in an overall response rate (OR) of 65% (90% CI 49.2%-75.7%) to date. VGPR was reached in 5 patients, PR in 17 patients, MR in 3 patients, SD in 4 patients and PD in 3. In patients double-refractory for proteasome inhibitors and lenalidomide, the OR was 69%, in patients with poor prognosis cytogenetic abnormalities it was 77%. The OR was independent from the number of prior therapy lines (OR rate 69% with < 5 prior therapy lines, OR rate 61% with ≥ 5 prior lines). Most frequent > grade (G) 2 adverse events to date were anemia (G3 29%), thrombocytopenia (G3 24%, G4 18%), infections (G3 24%, G4 9%, G5 3%), hyperglycemia (G3 18%, G4 3%) and fatigue (G3 12%). Six patients maintained their PR or better for the full 6 cycles per protocol while on study. Four patients continued NVd therapy on a compassionate use basis after completing the study. Updated final data will be provided at the meeting.
Conclusion: Nelfinavir in combination with bortezomib and dexamethasone (NVd) is a reasonable, active, safe and widely available treatment option for patients with proteasome inhibitor-refractory multiple myeloma. The objective response rate of 65% observed in this very advanced, heavily pretreated, mostly dual-refractory patient population is exceptional. Our results warrant further development of nelfinavir as a sensitizing drug for proteasome inhibitor-based treatments and promising new agent for MM therapy.
Figure 1. Maximum relative change in serum M-protein or serum free light chain concentration in individual evaluable patients.
Disclosures: Driessen: Mundipharma-EDO: Honoraria, Membership on an entity's Board of Directors or advisory committees; celgene: Consultancy; janssen: Consultancy. Samaras: Celgene (Adboard, educational talk), Amgen (adboard), Takeda (Adboard), Roche (Adboard), Sanofi (Adboard), Novartis (Adboard): Consultancy, Honoraria. Zander: Bristol Myers, Celgene, Amgen, Mundipharma, Janssen-Cilag, Takeda Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Gerelateerde artikelen
- ASCO 2021: aanbevolen abstracten voor vormen van bloedkanker, zoals vormen van leukemie en kanker van het beenmerg zoals Multiple Myeloma
- Belantamab mafodotin, pomalidomide en dexamethasone geeft betere ziekteprogressievrije tijd in vergelijking met pomalidomide, bortezomib en dexamethasone bij gevorderde multiple myeloma (ziekte van Kahler, botkanker)
- Bortezomib toegevoegd aan lenalidomide plus dexamethason bij niet eerder behandelde multiple myeloma geeft verbetering van progressievrije ziekte (plus en mediane overall overleving
- CAR-T cel behandeling met Ciltacabtagene autoleucel (cilta-cel-infusie) geeft lager risico op ziekteprogressie of overlijden en meer complete remissies dan standaardzorg bij patiënten met multiple myeloma met lenalidomide resistentie
- Cryotherapie tijdens toediening van Melphalan bij Kahler zorgt voor hoog significante verbetering van minder optreden van mucositis en andere bijwerkingen behorend bij chemo en beenmergtransplantatie.
- Daratumumab alleen gegeven geeft uitstekende resultaten bij patienten met actieve sluimerende Multiple Myeloma in vergelijking met actief monitoren
- Elotuzumab - een immuuntherapeutisch middel - aanvullend op lenalidomide en dexamethason geeft 30% betere overall overleving en progressievrije ziekte dan alleen lenalidomide en dexamethason bij gevorderde Multiple Myeloma - Kahler
- Genentest blijkt voorspellende waarde te hebben bij het verloop van Multiple Myeloma - ziekte van Kahler. Ook bij eerste diagnose.
- Hoewel overall overleving sterk verbetert met nieuwe medicijnen bij Multiple Myeloma / ziekte van Kahler blijven patienten met de del17p mutatie een veel slechtere prognose op overleving te hebben.
- Idecabtagene vicleucel (Abecma) geeft als immuuntherapie via CAR-T cellen bij patienten met zwaarvoorbehandelde vergevorderde multiple myeloma uitstekende resultaten copy 1
- intraveneus immunoglobuline voorkomt voor 90 procent ernstige infecties bij patienten met multiple myeloma die met op BCMA gerichte bispecifieke antilichamen worden behandeld
- Immuuntherapie met gepersonaliseerde dendritische celtherapie plus onderhoudsdosis lenalidomide na autologe stamceltransplantatie verbetert bij multiple myeloma complete remissies met ruim 7 procent na 1 jaar.
- Isatuximab aanvullend op lenalidomide, bortezomib en dexamethason vooraf aan stamceltransplantatie geeft betere meetbare restziekte - MRD (50 vs 36 procent) bij patiënten met nieuw gediagnosticeerde multiple myeloma die stamceltransplantatie ondergaan
- Isatuximab naast pomalidomide + dexamethason geeft betere overall overleving (Mediaan + 6,9 maanden) dan alleen pomalidomide + dexamethason bij patienten met multiple myeoloma (Kahler - botkanker) die zwaar voorbehandeld waren
- Jongere volwassenen met nieuw gediagnosticeerde multiple myeloma - botkanker die een autologe stamceltransplantatie ondergaan hebben een veel betere progressievrije overleving en een veel betere mediane overleving in vergelijking met 13 jaar geleden
- lenalidomide na autologe stamceltransplantatie bij multiple myeloma - ziekte van Kahler geeft superieure overall overleving en ziektevrije tijd
- Multiple Myeloma patienten met hoog risico vastgesteld door genentest hebben betere overleving bij behandeling met daratumumab, cyclofosfamide, bortezomib, lenalidomide en dexamethason zowel voor als na autologe stamceltransplantatie
- Meta-analysis Shows Association of Minimal Residual Disease–Negative Status With Improved Survival in Multiple Myeloma
- Nederlandse studie geeft overzicht van overall overleving bij Multiple Myeloma (Kahler) in alle stadia in periode 2008 tot 2013.
- Nelfinavir, een HIV remmer, plus bortezomib (velcade) geeft uitstekende resultaten - 65 procent bereikte PR - bij zwaar voorbehandelde patienten met vergevorderde multiple myeloma, Kahler
- Pembrolizumab plus lenalidomide en dexamethasone als eerstelijns behandeling voor patienten met nog onbehandelde multiple myeloma (Kahler) geeft geen betere resultaten en veel meer ernstige bijwerkingen
- Talquetamab, een nieuw bispecifiek antilichaam gericht op het antigen GPRC5D geeft veelbelovende resultaten bij zwaar voorbehandelde patienten met myeloma - botkanker (Kahler)
- Thalidomide naast dexamethason geeft bij Kahler - Multiple Myeloma een significant positief effect op aanslaan van behandeling, aldus Fase III studie. Artikel geplaatst juni 2004
- Teclistamab en daratumumab samen geeft bij gevorderde botkanker - multiple Myeloma betere progressievrije overleving en algehele overleving vergeleken met de standaardbehandeling
- Teclistamab geeft hele goede resultaten met 50 procent meer gedeeltelijke remissies en 20 procent complete remissies bij zwaar voorbehandelde patiënten met botkanker - multiple myeloma
- Vaccin gebaseerd op MUC1 lijkt veelbelovende ontwikkeling melden Israelische onderzoekers
- Velcade - Bortezomib: wat is dit voor middel en bij welke kankersoorten wordt het gebruikt: een overzicht van recente ontwikkelingen
- Venetoclax, een kleine molecuulremmer uit de BCL-2 groep, geeft veelbelovende resultaten bij vergevorderde zwaar voorbehandelde multiple myeloma (Kahler)
- Reguliere oncologie: Kahler - Multiple Myeloma - botkanker: een overzicht van recente ontwikkelingen binnen de reguliere oncologie bij elkaar gezet




Plaats een reactie ...
Reageer op "Nelfinavir, een HIV remmer, plus bortezomib (velcade) geeft uitstekende resultaten - 65 procent bereikte PR - bij zwaar voorbehandelde patienten met vergevorderde multiple myeloma, Kahler"