Zie ook in gerelateerde artikelen.
11 november 2019: met dank aan R. Hamers die ons op deze studie wees.
Aanvullend op onderstaande informatie lees ook dit studierapport: Omega-3 polyunsaturated fatty acid oral supplements for improving peripheral nerve health: a systematic review and meta-analysis
een reviewstudie over de waarde van visolie in het verminderen van neuropathie door chemo. Abstract onderaan artikel
9 maart 2016: Bron: Nutr Cancer. 2016 Jan;68(1):70-6. doi: 10.1080/01635581.2016.1115097. Epub 2015 Dec 23
Wanneer darmkankerpatiënten naast hun chemo 9 weken lang visolie krijgen dan ontstaat mediaan pas na 20 maanden (593 dagen) progressie van de ziekte terwijl patiënten die geen visolie kregen maar een placebo mediaan al na 11 maanden (330 dagen) progressie toonden van hun ziekte. Dit is bijna een verdubbeling van de ziekteprogressievrije tijd. (visoliegroep 593 dagen (±211.5) vs. placebogroep 330 dagen (± 135.1); P = 0.04]. Dit is echt spectaculair te noemen bij deze patientengroep. Ruim anderhalf jaar progressievrije ziekte komt bijna niet voor bij standaardbehandelingen voor uitgezaaide darmkanker.
Ook de CEA waarden daalden sneller en werden sneller en bleven langer lager in de visoliegroep dan in de placebogroep.
Ook een reviewstudie laat zien dat visolie zelfs een anti-PD effect kan hebben wanneer gebruikt naast gercihte behandelingen en / of naast chemo en/of bestraling bij vele verschillende vormen van kanker. Verderop in dit artikel de twee abstracten.
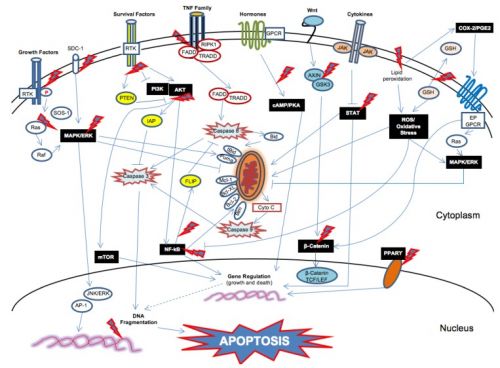
Deze studie kreeg ik toegestuurd door arts-bioloog drs. Engelbert Valstar met deze begeleidende tekst gericht aan prof. Voest, zie ook gerelateerde artikelen:
Wederom bewijs dat chemo (met o.a. oxaliplatin) juist gebaat is bij visolie ; dat Voest iets anders vindt in een muizenonderzoek (het andere is qua statistiek alleen goed voor de prullenbak) komt omdat hij de cisplatin oraal geeft ; visolie is ontstekingsremmend en ik denk dat daardoor de opname van cisplatin via de darm geremd wordt ; dit was bij voorbaat te verwachten (hij onderzoekt dus een artefact) ; waarom dan cisplatin bij muizen oraal geven, terwijl je weet dat dit bij de mens niet gebeurt en alle onderzoeken waarin dit niet gedaan is (nu in totaal 4 bij de mens en 3 bij proefdieren het tegenovergestelde) namelijk een gunstige interactie wordt gevonden.
Het is jammer dat het volledige studierapport: Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer niet gratis is te verkrijgen, of u moet er voor betalen.
Een andere studierapport is echter deze reviewstudie: Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy die bewijst dat visolie bij vele vormen van kanker en naast chemo en/of gerichte behandelingen voor goede resultaten zorgt. Zie ook de referentielijst van deze reviewstudie onderaan dit artikel
Hier de twee abstracten van de studies:
Supplementation with 2 g/day of fish oil for the first 9 wk of chemotherapy may contribute to delay in tumor progression in colorectal patients, possibly by enhancing the antineoplastic action of the chemotherapeutic drug.
Nutr Cancer. 2016 Jan;68(1):70-6. doi: 10.1080/01635581.2016.1115097. Epub 2015 Dec 23.
Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer.
Abstract
The authors evaluated clinical outcomes during and after chemotherapy in colorectal cancer patients supplemented with fish oil during the first 9 wk of treatment. Thirty individuals never submitted to chemotherapy were randomized into supplemented group (SG), which received 2 g/day of fish oil (0.6 g/day of EPA and DHA) for 9 wk or control group (CG), which received neither fish oil nor placebo. Outcomes assessed were number of chemotherapy cycles administered; days undergoing chemotherapy; number of delays and interruptions in the administration of chemotherapy; number of hospitalizations during chemotherapy; tumor progression; values of carcinoembryonic antigen (CEA); days until events (death and progression); and 3 yr survival. Time to tumor progression was significantly longer in SG [S593 days (±211.5)] vs. CG [330 days (± 135.1); P = 0.04], other outcomes did not differ between groups. Subjects with advanced cancer who received fish oil presented longer time to tumor progression and lower CEA values after chemotherapy; however these differences were not statistically significant. Supplementation with 2 g/day of fish oil for the first 9 wk of chemotherapy may contribute to delay in tumor progression in colorectal patients, possibly by enhancing the antineoplastic action of the chemotherapeutic drug.
- PMID:
- 26700096
- [PubMed - in process]
This analysis can allow a better comprehension of the potential cytotoxic therapeutic role of n-3 PUFAs against cancer, providing specific information and support to design future pre-clinical and clinical studies for a better use of n-3 PUFAs in cancer therapy, mainly combinational therapy.
J Clin Med. 2016 Feb; 5(2): 15.
Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy
Lindsay Brown, Academic Editor, Bernhard Rauch, Academic Editor, and Hemant Poudyal, Academic Editor
Abstract
Cancer is a major disease worldwide. Despite progress in cancer therapy, conventional cytotoxic therapies lead to unsatisfactory long-term survival, mainly related to development of drug resistance by tumor cells and toxicity towards normal cells. n-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can exert anti-neoplastic activity by inducing apoptotic cell death in human cancer cells either alone or in combination with conventional therapies. Indeed, n-3 PUFAs potentially increase the sensitivity of tumor cells to conventional therapies, possibly improving their efficacy especially against cancers resistant to treatment. Moreover, in contrast to traditional therapies, n-3 PUFAs appear to cause selective cytotoxicity towards cancer cells with little or no toxicity on normal cells. This review focuses on studies investigating the cytotoxic activity of n-3 PUFAs against cancer cells via apoptosis, analyzing the molecular mechanisms underlying this effective and selective activity. Here, we highlight the multiple molecules potentially targeted by n-3 PUFAs to trigger cancer cell apoptosis. This analysis can allow a better comprehension of the potential cytotoxic therapeutic role of n-3 PUFAs against cancer, providing specific information and support to design future pre-clinical and clinical studies for a better use of n-3 PUFAs in cancer therapy, mainly combinational therapy.
References
2.
Basile K.J., Aplin A.E. Resistance to chemotherapy: Short-term drug tolerance and stem cell-like subpopulations. Adv. Pharmacol. 2012;65:315–334. [PubMed]3.
Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [PubMed] [Cross Ref]4.
Maugeri-Saccà M., Vigneri P., de Maria R. Cancer stem cells and chemosensitivity. Clin. Cancer Res. 2011;17:4942–4947. doi: 10.1158/1078-0432.CCR-10-2538. [PubMed] [Cross Ref]5.
Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [PubMed] [Cross Ref]6.
Pritchard J.R., Bruno P.M., Gilbert L.A., Capron K.L., Lauffenburger D.A., Hemann M.T. Defining principles of combination drug mechanisms of action. Proc. Natl. Acad. Sci. USA. 2013;110:E170–E179. doi: 10.1073/pnas.1210419110. [PMC free article] [PubMed] [Cross Ref]7.
Burlingame B., Nishida C., Uauy R., Weisell R. Fats and fatty acids in human nutrition: Introduction. Ann. Nutr. Metable. 2009;55:5–7. doi: 10.1159/000228993. [PubMed] [Cross Ref]8.
Riediger N.D., Othman R.A., Suh M., Moghadasian M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009;109:668–679. doi: 10.1016/j.jada.2008.12.022. [PubMed] [Cross Ref]9.
Calder P.C. Marine ω-3 Fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [PubMed] [Cross Ref]10.
Gil A., Gil F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. Br. J. Nutr. 2015;113:S58–S67. doi: 10.1017/S0007114514003742. [PubMed] [Cross Ref]11.
Laviano A., Rianda S., Molfino A., Rossi Fanelli F. ω-3 Fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:156–161. doi: 10.1097/MCO.0b013e32835d2d99. [PubMed] [Cross Ref]12.
Bhagat U., Das U.N. Potential role of dietary lipids in the prophylaxis of some clinical conditions. Arch. Med. Sci. 2015;11:807–818. doi: 10.5114/aoms.2015.53302. [PMC free article] [PubMed] [Cross Ref]13.
Murray M., Hraiki A., Bebawy M., Pazderka C., Rawling T. Anti-tumor activities of lipids and lipid analogues and their development as potential anticancer drugs. Pharmacol. Ther. 2015;150:109–128. doi: 10.1016/j.pharmthera.2015.01.008. [PubMed] [Cross Ref]14.
Bang H.O., Dyerberg J., Nielsen A.B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;1:1143–1145. doi: 10.1016/S0140-6736(71)91658-8. [PubMed] [Cross Ref]15.
Gu Z., Shan K., Chen H., Chen Y.Q. n-3 Polyunsaturated fatty acids and their role in cancer chemoprevention. Curr. Pharmacol. Rep. 2015;5:283–294. doi: 10.1007/s40495-015-0043-9. [PMC free article] [PubMed] [Cross Ref]16.
Serini S., Fasano E., Piccioni E., Cittadini A.R., Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011;24:2093–2105. doi: 10.1021/tx200314p. [PubMed] [Cross Ref]17.
Chapkin R.S., DeClercq V., Kim E., Fuentes N.R., Fan Y.Y. Mechanisms by which pleiotropic amphiphilic n-3 PUFA reduce colon cancer risk. Curr. Colorectal Cancer Rep. 2014;10:442–452. doi: 10.1007/s11888-014-0241-6. [PMC free article] [PubMed] [Cross Ref]18.
Kiyabu G.Y., Inoue M., Saito E., Abe S.K., Sawada N., Ishihara J., Iwasaki M., Yamaji T., Shimazu T., et al. JPHC Study Group. Fish, n-3 polyunsaturated fatty acids and n-6 polyunsaturated fatty acids intake and breast cancer risk: The Japan Public Health Center-based prospective study. Int. J. Cancer. 2015;137:2915–2926. doi: 10.1002/ijc.29672. [PubMed] [Cross Ref]19.
Brasky T.M., Darke A.K., Song X., Tangen C.M., Goodman P.J., Thompson I.M., Meyskens F.L., Jr., Goodman G.E., Minasian L.M., et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2013;105:1132–1141. doi: 10.1093/jnci/djt174. [PMC free article] [PubMed] [Cross Ref]20.
Calder P.C., Deckelbaum R.J. Dietary fatty acids in health and disease: Greater controversy, greater interest. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:111–115. doi: 10.1097/MCO.0000000000000038. [PubMed] [Cross Ref]21.
Weylandt K.H., Serini S., Chen Y.Q., Su H.M., Lim K., Cittadini A., Calviello G. ω-3 Polyunsaturated fatty acids: The way forward in times of mixed evidence. Biomed. Res. Int. 2015;2015:143109. doi: 10.1155/2015/143109. [PMC free article] [PubMed] [Cross Ref]22.
Berquin I.M., Edwards I.J., Chen Y.Q. Multi-targeted therapy of cancer by ω-3 Fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [PMC free article] [PubMed] [Cross Ref]23.
Serini S., Piccioni E., Merendino N., Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis. 2009;14:132–152. doi: 10.1007/s10495-008-0298-2. [PubMed] [Cross Ref]24.
Gleissman H., Johnsen J.I., Kogner P. ω-3 Fatty acids in cancer, the protectors of good and the killers of evil? Exp. Cell Res. 2010;316:1365–1373. doi: 10.1016/j.yexcr.2010.02.039. [PubMed] [Cross Ref]25.
Vaughan V.C., Hassing M.R., Lewandowski P.A. Marine polyunsaturated fatty acids and cancer therapy. Br. J. Cancer. 2013;108:486–492. doi: 10.1038/bjc.2012.586. [PMC free article] [PubMed] [Cross Ref]26.
Biondo P.D., Brindley D.N., Sawyer M.B., Field C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008;19:787–796. doi: 10.1016/j.jnutbio.2008.02.003. [PubMed] [Cross Ref]27.
Siddiqui R.A., Harvey K.A., Xu Z., Bammerlin E.M., Walker C., Altenburg J.D. Docosahexaenoic acid: A natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors. 2011;37:399–412. doi: 10.1002/biof.181. [PubMed] [Cross Ref]28.
Wang J., Luo T., Li S., Zhao J. The powerful applications of polyunsaturated fatty acids in improving the therapeutic efficacy of anticancer drugs. Expert Opin. Drug Deliv. 2012;9:1–7. doi: 10.1517/17425247.2011.618183. [PubMed] [Cross Ref]29.
Merendino N., Costantini L., Manzi L., Molinari R., D’Eliseo D., Velotti F. Dietary ω -3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. Biomed. Res. Int. 2013;2013:310186. doi: 10.1155/2013/310186. [PMC free article] [PubMed] [Cross Ref]30.
Hajjaji N., Bougnoux P. Selective sensitization of tumors to chemotherapy by marine-derived lipids: A review. Cancer Treat. Rev. 2013;39:473–488. doi: 10.1016/j.ctrv.2012.07.001. [PubMed] [Cross Ref]31.
de Aguiar Pastore Silva J., Emilia de Souza Fabre M., Waitzberg D.L. ω-3 Supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin. Nutr. 2015;34:359–366. doi: 10.1016/j.clnu.2014.11.005. [PubMed] [Cross Ref]32.
Das U.N., Madhavi N., Sravan Kumar G., Padma M., Sangeetha P. Can tumour cell drug resistance be reversed by essential fatty acids and their metabolites? Prostaglandins Leukot. Essent. Fatty Acids. 1998;58:39–54. doi: 10.1016/S0952-3278(98)90128-4. [PubMed] [Cross Ref]33.
Slagsvold J.E., Pettersen C.H., Størvold G.L., Follestad T., Krokan H.E., Schønberg S.A. DHA alters expression of target proteins of cancer therapy in chemotherapy resistant SW620 colon cancer cells. Nutr. Cancer. 2010;62:611–621. doi: 10.1080/01635580903532366. [PubMed] [Cross Ref]34.
Kuan C.Y., Walker T.H., Luo P.G., Chen C.F. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J. Am. Coll. Nutr. 2011;30:265–273. doi: 10.1080/07315724.2011.10719969. [PubMed] [Cross Ref]35.
Gelsomino G., Corsetto P.A., Campia I., Montorfano G., Kopecka J., Castella B., Gazzano E., Ghigo D., Rizzo A.M., Riganti C. ω 3 Fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol. Cancer. 2013;12:137. doi: 10.1186/1476-4598-12-137. [PMC free article] [PubMed] [Cross Ref]36.
Das U.N., Begin M.E., Ells G., Huang Y.S., Horrobin D.F. Polyunsaturated fatty acids augment free radical generation in tumor cells in vitro. Biochem. Biophys. Res. Commun. 1987;145:15–24. doi: 10.1016/0006-291X(87)91281-2. [PubMed] [Cross Ref]37.
Tsai W.S., Nagawa H., Kaizaki S., Tsuruo T., Muto T. Inhibitory effects of n--3 polyunsaturated fatty acids on sigmoid colon cancer transformants. J. Gastroenterol. 1998;33:206–212. doi: 10.1007/s005350050071. [PubMed] [Cross Ref]38.
Siddiqui R.A., Harvey K., Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem. Phys. Lipids. 2008;153:47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [PubMed] [Cross Ref]39.
Giros A., Grzybowski M., Sohn V.R., Pons E., Fernandez-Morales J., Xicola R.M., Sethi P., Grzybowski J., Goel A., et al. Regulation of colorectal cancer cell apoptosis by the n-3 polyunsaturated fatty acids Docosahexaenoic and Eicosapentaenoic. Cancer Prev. Res. 2009;2:732–742. doi: 10.1158/1940-6207.CAPR-08-0197. [PMC free article] [PubMed] [Cross Ref]40.
Toit-Kohn J.L., Louw L., Engelbrecht A.M. Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J. Nutr. Biochem. 2009;20:106–114. doi: 10.1016/j.jnutbio.2007.12.005. [PubMed] [Cross Ref]41.
Gleissman H., Segerström L., Hamberg M., Ponthan F., Lindskog M., Johnsen J.I., Kogner P. ω-3 Fatty acid supplementation delays the progression of neuroblastoma in vivo. Int. J. Cancer. 2011;128:1703–1711. doi: 10.1002/ijc.25473. [PubMed] [Cross Ref]42.
Nikolakopoulou Z., Nteliopoulos G., Michael-Titus A.T., Parkinson E.K. ω-3 Polyunsaturated fatty acids selectively inhibit growth in neoplastic oral keratinocytes by differentially activating ERK1/2. Carcinogenesis. 2013;34:2716–2725. doi: 10.1093/carcin/bgt257. [PMC free article] [PubMed] [Cross Ref]43.
Ravacci G.R., Brentani M.M., Tortelli T.Jr., Torrinhas R.S., Saldanha T., Torres E.A., Waitzberg D.L. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J. Nutr. Biochem. 2013;24:505–515. doi: 10.1016/j.jnutbio.2012.02.001. [PubMed] [Cross Ref]44.
Abdi J., Garssen J., Faber J., Redegeld F.A. ω-3 Fatty acids, EPA and DHA induce apoptosis and enhance drug sensitivity in multiple myeloma cells but not in normal peripheral mononuclear cells. J. Nutr. Biochem. 2014;25:1254–1262. doi: 10.1016/j.jnutbio.2014.06.013. [PubMed] [Cross Ref]45.
Berstad P., Thiis-Evensen E., Vatn M.H., Almendingen K. Fatty acids in habitual diet, plasma phospholipids, and tumour and normal colonic biopsies in young colorectal cancer patients. J. Oncol. 2012;2012:254801. doi: 10.1155/2012/254801. [PMC free article] [PubMed] [Cross Ref]46.
Thomas G.C. Apoptosis and cancer: The genesis of a research field. Nature Rev. Cancer. 2009;9:501–507. [PubMed]47.
Logue S.E., Gorman A.M., Cleary P., Keogh N., Samali A. Current concepts in ER stress-induced apoptosis. J. Carcinogene Mutagene. 2013 doi: 10.4172/2157-2518.S6-002. [Cross Ref]48.
Mengeaud V., Nano J.L., Fournel S., Rampal P. Effects of eicosapentaenoic acid, γ-linolenic acid and prostaglandin E1 on three human colon carcinoma cell lines. Prostaglandins Leukot. Essent. Fatty Acids. 1992;47:313–319. doi: 10.1016/0952-3278(92)90204-V. [PubMed] [Cross Ref]49.
Clarke R.G., Lund E.K., Latham P., Pinder A.C., Johnson I.T. Effect of eicosapentaenoic acid on the proliferation and incidence of apoptosis in the colorectal cell line HT29. Lipids. 1999;34:1287–1295. doi: 10.1007/s11745-999-0480-7. [PubMed] [Cross Ref]50.
Chen Z.Y., Istfan N.W. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:301–308. doi: 10.1054/plef.2000.0218. [PubMed] [Cross Ref]51.
Kubota H., Matsumoto H., Higashida M., Murakami H., Nakashima H., Oka Y., Okumura H., Yamamura M., Nakamura M., Hirai T. Eicosapentaenoic acid modifies cytokine activity and inhibits cell proliferation in an oesophageal cancer cell line. Anticancer Res. 2013;33:4319–4324. [PubMed]52.
Lee S.E., Lim J.W., Kim H. Activator protein-1 mediates docosahexaenoic acid-induced apoptosis of human gastric cancer cells. Ann. N. Y. Acad. Sci. 2009;1171:163–169. doi: 10.1111/j.1749-6632.2009.04716.x. [PubMed] [Cross Ref]53.
Lim K., Han C., Dai Y., Shenm M., Wu T. ω-3 Polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking β-catenin and cyclooxygenase-2. Mol. Cancer Ther. 2009;8:3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [PMC free article] [PubMed] [Cross Ref]54.
Sun S.N., Jia W.D., Chen H., Ma J.L., Ge Y.S., Yu J.H., Li J.S. Docosahexaenoic acid (DHA) induces apoptosis in human hepatocellular carcinoma cells. Int. J. of Clin. Exp. Pathol. 2013;6:281–289. [PMC free article] [PubMed]55.
Zhang Y., Han L., Qi W., Cheng D., Ma X., Hou L., Cao X., Wang C. Eicosapentaenoic acid (EPA) induced apoptosis in HepG2 cells through ROS-Ca2+-JNK mitochondrial pathways. Biochem. Biophys. Res. Commun. 2015;456:926–932. doi: 10.1016/j.bbrc.2014.12.036. [PubMed] [Cross Ref]56.
Hawkins R.A., Sangster K., Arends M.J. Apoptotic death of pancreatic cancer cells induced by polyunsaturated fatty acids varies with double bond number and involves an oxidative mechanism. J. Pathol. 1998;185:61–70. doi: 10.1002/(SICI)1096-9896(199805)185:1<61::AID-PATH49>3.0.CO;2-8. [PubMed] [Cross Ref]57.
Merendino N., Loppi B., D’Aquino M., Molinari R., Pessina G., Romano C., Velotti F. Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation. Nutr. Cancer. 2005;52:225–233. doi: 10.1207/s15327914nc5202_12. [PubMed] [Cross Ref]58.
Fukui M., Kang K.S., Okada K., Zhu B.T. EPA, an ω-3 Fatty acid, induces apoptosis in human pancreatic cancer cells: Role of ROS accumulation, caspase-8 activation, and autophagy induction. J. Cell. Biochem. 2013;114:192–203. doi: 10.1002/jcb.24354. [PubMed] [Cross Ref]59.
Lim K., Han C., Xu L., Isse K., Demetris A.J., Wu T. Cyclooxygenase-2-derived prostaglandin E2 activates β-catenin in human cholangiocarcinoma cells: Evidence for inhibition of these signaling pathways by ω 3 polyunsaturated fatty acids. Cancer Res. 2008;68:553–560. doi: 10.1158/0008-5472.CAN-07-2295. [PubMed] [Cross Ref]60.
Rose D.P., Connolly J.M. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990;50:7139–7144. [PubMed]61.
Chamras H., Ardashian A., Heber D., Glaspy J.A. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J. Nutr. Biochem. 2002;13:711–716. doi: 10.1016/S0955-2863(02)00230-9. [PubMed] [Cross Ref]62.
Sharma A., Belna J., Logan J., Espat J., Hurteau J.A. The effects of ω-3 fatty acids on growth regulation of epithelial ovarian cancer cell lines. Gynecol. Oncol. 2005;99:58–64. doi: 10.1016/j.ygyno.2005.05.024. [PubMed] [Cross Ref]63.
Narayanan N.K., Narayanan B.A., Reddy B.S. A combination of docosahexaenoic acid and celecoxib prevents prostate cancer cell growth in vitro and is associated with modulation of nuclear factor-κB, and steroid hormone receptors. Int. J. Oncol. 2005;26:785–792. doi: 10.3892/ijo.26.3.785. [PubMed] [Cross Ref]64.
Hu Y., Sun H., Owens R.T., Gu Z., Wu J., Chen Y.Q., O’Flaherty J.T., Edwards I.J. Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia. 2010;12:826–836. doi: 10.1593/neo.10586. [PMC free article] [PubMed] [Cross Ref]65.
Molinari R., D’Eliseo D., Manzi L., Zolla L., Velotti F., Merendino N. The n3-polyunsaturated fatty acid docosahexaenoic acid induces immunogenic cell death in human cancer cell lines via pre-apoptotic calreticulin exposure. Cancer Immunol. Immunother. 2011;60:1503–1507. doi: 10.1007/s00262-011-1074-7. [PubMed] [Cross Ref]66.
Lindskog M., Gleissman H., Ponthan F., Castro J., Kogner P., Johnsen J.I. Neuroblastoma cell death in response to docosahexaenoic acid: Sensitization to chemotherapy and arsenic induced oxidative stress. Int. J. Cancer. 2006;118:2584–2593. doi: 10.1002/ijc.21555. [PubMed] [Cross Ref]67.
Faragó N., Fehér L.Z., Kitajka K., Das U.N., Puskás L.G. MicroRNA profile of polyunsaturated fatty acid treated glioma cells reveal apoptosis-specific expression changes. Lipids Health Dis. 2011;10:173. doi: 10.1186/1476-511X-10-173. [PMC free article] [PubMed] [Cross Ref]68.
Serini S., Trombino S., Oliva F., Piccioni E., Monego G., Resci F., Boninsegna A., Picci N., Ranelletti F.O., Calviello G. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis. 2008;13:1172–1183. doi: 10.1007/s10495-008-0246-1. [PubMed] [Cross Ref]69.
Yao Q.H., Zhang X.C., Fu T., Gu J.Z., Wang L., Wang Y., Lai Y.B., Wang Y.Q., Guo Y. ω-3 polyunsaturated fatty acids inhibit the proliferation of the lung adenocarcinoma cell line A549 in vitro. Mol. Med. Rep. 2014;9:401–406. [PubMed]70.
Albino A.P., Juan G., Traganos F., Reinhart L., Connolly J., Rose D.P., Darzynkiewicz Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: Association with decreased pRb phosphorylation. Cancer Res. 2000;60:4139–4145. [PubMed]71.
Denkins Y., Kempf D., Ferniz M., Nileshwar S., Marchetti D. Role of ω-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolism in brain-metastatic melanoma. J. Lipid Res. 2005;46:1278–1284. doi: 10.1194/jlr.M400474-JLR200. [PubMed] [Cross Ref]72.
Finstad H.S., Myhrstad M.C., Heimli H., Lømo J., Blomhoff H.K., Kolset S.O., Drevon C.A. Multiplication and death-type of leukemia cell lines exposed to very long-chain polyunsaturated fatty acids. Leukemia. 1998;12:921–929. doi: 10.1038/sj.leu.2401030. [PubMed] [Cross Ref]73.
Finstad H.S., Drevon C.A., Kulseth M.A., Synstad A.V., Knudsen E., Kolset S.O. Cell proliferation, apoptosis and accumulation of lipid droplets in U937-1 cells incubated with eicosapentaenoic acid. Biochem. J. 1998;336:451–459. doi: 10.1042/bj3360451. [PMC free article] [PubMed] [Cross Ref]74.
Chiu L.C.M., Wan J.M.F. Induction of apoptosis in HL-60 cells by eicosapentaenoic acid (EPA) is associated with downregulation of BCL-2 expression. Cancer Letters. 1999;145:17–27. doi: 10.1016/S0304-3835(99)00224-4. [PubMed] [Cross Ref]75.
Chiu L.C., Wong E.Y., Ooi V.E. Docosahexaenoic acid modulates different genes in cell cycle and apoptosis to control growth of human leukemia HL-60 cells. Int J Oncol. 2004;25:737–744. doi: 10.3892/ijo.25.3.737. [PubMed] [Cross Ref]76.
Siddiqui R.A., Jenski L.J., Neff K., Harvey K., Kovacs R.J., Stillwell W. Docosahexaenoic acid induces apoptosis in Jurkat cells by a protein phosphatase-mediated process. Biochim. Biophys. Acta. 2001;1499:265–275. doi: 10.1016/S0167-4889(00)00128-2. [PubMed] [Cross Ref]77.
Zand H., Rhimipour A., Bakhshayesh M., Shafiee M., Nour Mohammadi I., Salimi S. Involvement of PPAR-γ and p53 in DHA-induced apoptosis in Reh cells. Mol. Cell. Biochem. 2007;304:71–77. doi: 10.1007/s11010-007-9487-5. [PubMed] [Cross Ref]78.
Yamagami T., Porada C.D., Pardini R.S., Zanjani E.D., Almeida-Porada G. Docosahexaenoic acid induces dose dependent cell death in an early undifferentiated subtype of acute myeloid leukemia cell line. Cancer Biol. Ther. 2009;8:331–337. doi: 10.4161/cbt.8.4.7334. [PMC free article] [PubMed] [Cross Ref]79.
Sravan Kumar G., Das U.N. Cytotoxic action of α-linolenic and eicosapentaenoic acids on myeloma cells in vitro. Prostaglandins Leukot. Essent. Fatty Acids. 1997;56:285–293. doi: 10.1016/S0952-3278(97)90572-X. [PubMed] [Cross Ref]80.
Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., de Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [PubMed] [Cross Ref]81.
Das U.N. Essential fatty acids and their metabolites as modulators of stem cell biology with reference to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2011;30:311–324. doi: 10.1007/s10555-011-9316-x. [PubMed] [Cross Ref]82.
Yang T., Fang S., Zhang H.X., Xu L.X., Zhang Z.Q., Yuan K.T., Xue C.L., Yu H.L., Zhang S., Li Y.F., et al. n-3 PUFA shave antiproliferative and apoptotic effects on human colorectal cancer stemlike cells in vitro. J. Nutr. Biochem. 2013;24:744–753. doi: 10.1016/j.jnutbio.2012.03.023. [PubMed] [Cross Ref]83.
Vasudevan A., Yu Y., Banerjee S., Woods J., Farhana L., Rajendra S.G., Patel A., Dyson G., Levi E., Maddipati K.R., et al. ω-3 Fatty acid is a potential preventive agent for recurrent colon cancer. Cancer Prev. Res. 2014;7:1138–1148. doi: 10.1158/1940-6207.CAPR-14-0177. [PMC free article] [PubMed] [Cross Ref]84.
De Carlo F., Witte T.R., Hardman W.E., Claudio P.P. ω-3 Eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy. PLoS ONE. 2013;8:15 [PMC free article] [PubMed]85.
Xiong A., Yu W., Liu Y., Sanders B.G., Kline K. Elimination of ALDH+ breast tumor initiating cells by docosahexanoic acid and/or γ tocotrienol through SHP-1 inhibition of Stat3 signaling. Mol. Carcinog. 2015 doi: 10.1002/mc.22291. [PubMed] [Cross Ref]86.
Rose D.P., Connolly J.M., Rayburn J., Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl. Cancer Inst. 1995;87:587–592. doi: 10.1093/jnci/87.8.587. [PubMed] [Cross Ref]87.
Yam D., Peled A., Huszar M., Shinitzky M. Dietary fish oil suppresses tumor growth and metastasis of Lewis lung carcinoma in mice. J. Nutr. Biochem. 1997;8:619–622. doi: 10.1016/S0955-2863(97)00089-2. [Cross Ref]88.
Boudreau M.D., Sohn K.H., Rhee S.H., Lee S.W., Hunt J.D., Hwang D.H. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: Mediation through cyclooxygenase-independent pathways. Cancer Res. 2001;61:1386–1391. [PubMed]89.
Kato T., Hancock R.L., Mohammadpour H., McGregor B., Manalo P., Khaiboullina S., Hall M.R., Pardini L., Pardini R.S. Influence of ω-3 fatty acids on the growth of human colon carcinoma in nude mice. Cancer Lett. 2002;187:169–177. doi: 10.1016/S0304-3835(02)00432-9. [PubMed] [Cross Ref]90.
Camargo C.Q., Mocellin M.C., Pastore Silva J.A., de Souza Fabre M.E., Nunes E.A., de Moraes Trinidade E.B. Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer. Nutr. Cancer. [(accessed on 19 January 2016)]. (in press) Available online: http://.doi.org/10.1080/01635581.2016.1115097. [PubMed]91.
Bougnoux P., Hajjaji N., Ferrasson M.N., Giraudeau B., Couet C., le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer. 2009:1011978–1011985. doi: 10.1038/sj.bjc.6605441. [PMC free article] [PubMed] [Cross Ref]92.
Cockbain J., Toogood G.J., Hull M.A. ω-3 Polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [PubMed] [Cross Ref]93.
Murphy R.A., Mourtzakis M., Chu Q.S., Baracos V.E., Reiman T., Mazurak V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced non-small cell lung cancer. Cancer. 2011;117:3774–3780. doi: 10.1002/cncr.25933. [PubMed] [Cross Ref]94.
Patterson R.E., Flatt S.W., Newman V.A., Natarajan L., Rock C.L., Thomson C.A., Caan B.J., Parker B.A., Pierce J.P. Marine fatty acid intake is associated with breast cancer prognosis. J. Nutr. 2011;141:201–206. doi: 10.3945/jn.110.128777. [PMC free article] [PubMed] [Cross Ref]95.
Cockbain A.J., Volpato M., Race A.D., Munarini A., Fazio C., Belluzzi A., Loadman P.M., Toogood G.J., Hull M.A. Anticolorectal cancer activity of the ω-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014;63:1760–1768. doi: 10.1136/gutjnl-2013-306445. [PubMed] [Cross Ref]96.
Sánchez-Lara K., Turcott J.G., Juárez-Hernández E., Nuñez-Valencia C., Villanueva G., Guevara P., de la Torre-Vallejo M., Mohar A., Arrieta O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014;33:1017–1023. doi: 10.1016/j.clnu.2014.03.006. [PubMed] [Cross Ref]97.
Arshad A., Chung W.Y., Isherwood J., Mann C.D., Al-Leswas D., Steward W.P., Metcalfe M.S., Dennison A.R. Cellular and plasma uptake of parenteral ω-3 rich lipid emulsion fatty acids in patients with advanced pancreatic cancer. Clin. Nutr. 2014;33:895–899. doi: 10.1016/j.clnu.2013.09.017. [PubMed] [Cross Ref]98.
Ma Y.J., Yu J., Xiao J., Cao B.W. The consumption of ω-3 polyunsaturated fatty acids improves clinical outcomes and prognosis in pancreatic cancer patients: A systematic evaluation. Nutr. Cancer. 2015;67:112–118. doi: 10.1080/01635581.2015.976315. [PubMed] [Cross Ref]99.
Nabavi S.F., Bilottom S., Russom G.L., Orhan I.E., Habtemariam S., Daglia M., Devi K.P., Loizzo M.R., Tundis R., Nabavi S.M. ω-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34:359–380. doi: 10.1007/s10555-015-9572-2. [PubMed] [Cross Ref]100.
Khankari N.K., Bradshaw P.T., Steck S.E., He K., Olshan A.F., Shen J., Ahn J., Chen Y., Ahsan H., Terry M.B., et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer. 2015 doi: 10.1002/cncr.29329. [PMC free article] [PubMed] [Cross Ref]101.
Mocellin M.C., Camargo C.Q., Nunes E.A., Fiates G.M., Trindade E.B. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin. Nutr. 2015 doi: 10.1016/j.clnu.2015.04.013. [PubMed] [Cross Ref]102.
Heimli H., Giske C., Naderi S., Drevon C.A., Hollung K. Eicosapentaenoic acid promotes apoptosis in Ramos cells via activation of caspase-3 and -9. Lipids. 2002;37:797–802. doi: 10.1007/s11745-002-0963-6. [PubMed] [Cross Ref]103.
Llor X., Pons E., Roca A., Alvarez M., Mañé J., Fernández-Bañares F., Gassull M.A. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 2003;22:71–79. doi: 10.1054/clnu.2002.0627. [PubMed] [Cross Ref]104.
Danbara N., Yuri T., Tsujita-Kyutoku M., Sato M., Senzaki H., Takada H., Hada T., Miyazawa T., Okazaki K., Tsubura A. Conjugated docosahexaenoic acid is a potent inducer of cell cycle arrest and apoptosis and inhibits growth of colo 201 human colon cancer cells. Nutr. Cancer. 2004;50:71–79. doi: 10.1207/s15327914nc5001_10. [PubMed] [Cross Ref]105.
Calviello G., Di Nicuolo F., Serini S., Piccioni E., Boninsegna A., Maggiano N., Ranelletti F.O., Palozza P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother. Pharmacol. 2005;55:12–20. doi: 10.1007/s00280-004-0846-6. [PubMed] [Cross Ref]106.
Jakobsen C.H., Størvold G.L., Bremseth H., Follestad T., Sand K., Mack M., Olsen K.S., Lundemo A.G., Iversen J.G., Krokan H.E., et al. DHA induces ER stress and growth arrest in human colon cancer cells: Associations with cholesterol and calcium homeostasis. J. Lipid. Res. 2008;49:2089–2100. doi: 10.1194/jlr.M700389-JLR200. [PMC free article] [PubMed] [Cross Ref]107.
Arita K., Kobuchi H., Utsumi T., Takehara Y., Akiyama J., Horton A.A., Utsumi K. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem. Pharmacol. 2001;62:821–828. doi: 10.1016/S0006-2952(01)00723-7. [PubMed] [Cross Ref]108.
Narayanan B.A., Narayanan N.K., Reddy B.S. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int. J. Oncol. 2001;19:1255–1262. doi: 10.3892/ijo.19.6.1255. [PubMed] [Cross Ref]109.
Kolch W., Halasz M., Granovskaya M., Kholodenko B.N. The dynamic control of signal transduction networks in cancer cells. Nat. Rev. Cancer. 2015;15:515–527. doi: 10.1038/nrc3983. [PubMed] [Cross Ref]110.
Huang C.Y., Yu L.C. Pathophysiological mechanisms of death resistance in colorectal carcinoma. World J. Gastroenterol. 2015;21:11777–11792. doi: 10.3748/wjg.v21.i41.11777. [PMC free article] [PubMed] [Cross Ref]111.
Glatz J.F., Luiken J.J., van Nieuwenhoven F.A., van der Vusse G.J. Molecular mechanism of cellular uptake and intracellular translocation of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 1997;57:3–9. doi: 10.1016/S0952-3278(97)90485-3. [PubMed] [Cross Ref]112.
Wassall S.R., Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: Domain formation in membranes. Biochim. Biophys. Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [PubMed] [Cross Ref]113.
Zhang C., Yu H., Ni X., Shen S., Das U.N. Growth inhibitory effect of polyunsaturated fatty acids (PUFAs) on colon cancer cells via their growth inhibitory metabolites and fatty acid composition changes. PLoS ONE. 2015;10:15 [PMC free article] [PubMed]114.
Ibarguren M., López D.J., Escribá P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta. 2014;1838:1518–1528. doi: 10.1016/j.bbamem.2013.12.021. [PubMed] [Cross Ref]115.
Corsetto P.A., Cremona A., Montorfano G., Jovenitti I.E., Orsini F., Arosio P., Rizzo A.M. Chemical-physical changes in cell membrane microdomains of breast cancer cells after ω-3 PUFA incorporation. Cell Biochem. Biophys. 2012;64:45–59. doi: 10.1007/s12013-012-9365-y. [PubMed] [Cross Ref]116.
Schley P.D., Brindley D.N., Field C.J. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 2007;137:548–553. [PubMed]117.
Rogers K.R., Kikawa K.D., Mouradian M., Hernandez K., McKinnon K.M., Ahwah S.M., Pardini R.S. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31:1523–1530. doi: 10.1093/carcin/bgq111. [PubMed] [Cross Ref]118.
Corsetto P.A., Montorfano G., Zava S., Jovenitti I.E., Cremona A., Berra B., Rizzo A.M. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011;10:73. doi: 10.1186/1476-511X-10-73. [PMC free article] [PubMed] [Cross Ref]119.
Lee E.J., Yun U.J., Koo K.H., Sung J.Y., Shim J., Ye S.K., Hong K.M., Kim Y.N. Down-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim. Biophys. Acta. 2014;1841:190–203. doi: 10.1016/j.bbalip.2013.10.006. [PubMed] [Cross Ref]120.
Mason J.K., Klaire S., Kharotia S., Wiggins A.K., Thompson L.U. α-linolenic acid and docosahexaenoic acid, alone and combined with trastuzumab, reduce HER2-overexpressing breast cancer cell growth but differentially regulate HER2 signaling pathways. Lipids Health Dis. 2015;14:91. doi: 10.1186/s12944-015-0090-6. [PMC free article] [PubMed] [Cross Ref]121.
Cao W., Ma Z., Rasenick M.M., Yeh S., Yu J. n-3 poly-unsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth. PLoS ONE. 2012;7:15 doi: 10.1371/journal.pone.0052838. [PMC free article] [PubMed] [Cross Ref]122.
Ewaschuk J.B., Newell M., Field C.J. Docosahexanoic acid improves chemotherapy efficacy by inducing CD95 translocation to lipid rafts in ER− breast cancer cells. Lipids. 2012;47:1019–1030. doi: 10.1007/s11745-012-3717-7. [PubMed] [Cross Ref]123.
Gu Z., Wu J., Wang S., Suburu J., Chen H., Thomas M.J., Shi L., Edwards I.J., Berquin I.M., Chen Y.Q. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis. 2013;34:1968–1975. doi: 10.1093/carcin/bgt147. [PMC free article] [PubMed] [Cross Ref]124.
Calviello G., Resci F., Serini S., Piccioni E., Toesca A., Boninsegna A., Monego G., Ranelletti F.O., Palozza P. Docosahexaenoic acid induces proteasome-dependent degradation of β-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis. 2007;28:1202–1209. doi: 10.1093/carcin/bgl254. [PubMed] [Cross Ref]125.
Song K.S., Jing K., Kim J.S., Yun E.J., Shin S., Seo K.S., Park J.H., Heo J.Y., Kang J.X., Suh K.S., et al. ω-3-Polyunsaturated fatty acids suppress pancreatic cancer cell growth in vitro and in vivo via downregulation of Wnt/β-catenin signaling. Pancreatology. 2011;11:574–584. doi: 10.1159/000334468. [PubMed] [Cross Ref]126.
Xue M., Wang Q., Zhao J., Dong L., Ge Y., Hou L., Liu Y., Zheng Z. Docosahexaenoic acid inhibited the Wnt/β-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J. Nutr. Biochem. 2014;25:104–110. doi: 10.1016/j.jnutbio.2013.09.008. [PubMed] [Cross Ref]127.
Sun H., Hu Y., Gu Z., Owens R.T., Chen Y.Q., Edwards I.J. ω-3 Fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecan-1 inhibition of the MEK-Erk pathway. Carcinogenesis. 2011;32:1518–1524. doi: 10.1093/carcin/bgr132. [PMC free article] [PubMed] [Cross Ref]128.
Schley P.D., Jijon H.B., Robinson L.E., Field C.J. Mechanisms of ω-3 fatty acid-induced growth inhibition in MDAMB-231 human breast cancer cells. Breast Cancer Res. Treat. 2005;92:187–195. doi: 10.1007/s10549-005-2415-z. [PubMed] [Cross Ref]129.
Ghosh-Choudhury T., Mandal C.C., Woodruff K., St Clair P., Fernandes G., Choudhury G.G., Ghosh-Choudhury N. Fish oil targets PTEN to regulate NFκB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res. Treat. 2009;118:213–228. doi: 10.1007/s10549-008-0227-7. [PMC free article] [PubMed] [Cross Ref]130.
Engelbrecht A.M., Toit-Kohn J.L., Ellis B., Thomas M., Nell T., Smith R. Differential induction of apoptosis and inhibition of the PI3-kinase pathway by saturated, monounsaturated and polyunsaturated fatty acids in a colon cancer cell model. Apoptosis. 2008;13:1368–1377. doi: 10.1007/s10495-008-0260-3. [PubMed] [Cross Ref]131.
Jing K., Song K.S., Shin S., Kim N., Jeong S., Oh H.R., Park J.H., Seo K.S., Heo J.Y., Han J., et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy. 2011;7:1348–1358. doi: 10.4161/auto.7.11.16658. [PMC free article] [PubMed] [Cross Ref]132.
Shin S., Jing K., Jeong S., Kim N., Song K.S., Heo J.Y., Park J.H., Seo K.S., Han J., Park J.I., et al. The ω-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed. Res. Int. 2013;2013:568671. doi: 10.1155/2013/568671. [PMC free article] [PubMed] [Cross Ref]133.
Kim N., Jeong S., Jing K., Shin S., Kim S., Heo J.Y., Kweon G.R., Park S.K., Wu T., Park J.I., et al. Docosahexaenoic acid induces cell death in human non-small cell Lung cancer cells by repressing mTOR via AMPK activation and PI3K/Akt inhibition. Biomed. Res. Int. 2015;2015:239764. doi: 10.1155/2015/239764. [PMC free article] [PubMed] [Cross Ref]134.
Rescigno T., Capasso A., Tecce M.F. Effect of Docosahexaenoic acid on cell cycle pathways in Breast cell lines with different transformation degree. J. Cell. Physiol. 2015 doi: 10.1002/jcp.25217. [PubMed] [Cross Ref]135.
Shaikh I.A., Brown I., Schofield A.C., Wahle K.W., Heys S.D. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: The role of genes associated with the NF-κB pathway. Prostate. 2008;68:1635–1646. doi: 10.1002/pros.20830. [PubMed] [Cross Ref]136.
Cavazos D.A., Price R.S., Apte S.S., de Graffenried L.A. Docosahexaenoic acid selectively induces human prostate cancer cell sensitivity to oxidative stress through modulation of NF-κB. Prostate. 2011;71:1420–1428. doi: 10.1002/pros.21359. [PubMed] [Cross Ref]137.
Jeong S., Jing K., Kim N., Shin S., Kim S., Song K.S., Heo J.Y., Park J.H., Seo K.S., Han J., et al. Docosahexaenoic acid-induced apoptosis is mediated by activation of mitogen-activated protein kinases in human cancer cells. BMC Cancer. 2014;14:481. doi: 10.1186/1471-2407-14-481. [PMC free article] [PubMed] [Cross Ref]138.
Gleissman H., Yang R., Martinod K., Lindskog M., Serhan C.N., Johnsen J.I., Kogner P. Docosahexaenoic acid metabolome in neural tumors: Identification of cytotoxic intermediates. FASEB J. 2010;24:906–915. doi: 10.1096/fj.09-137919. [PMC free article] [PubMed] [Cross Ref]139.
Serhan C.N., Arita M., Hong S., Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel ω-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [PubMed] [Cross Ref]140.
Hong S., Lu Y., Yang R., Gotlinger K.H., Petasis N.A., Serhan C.N. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: Analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J. Am. Soc. Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [PMC free article] [PubMed] [Cross Ref]141.
Ding W.Q., Vaught J.L., Yamauchi H., Lind S.E. Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: The potential importance of down-regulation of superoxide dismutase 1 expression. Mol. Cancer Ther. 2004;3:1109–1117. [PubMed]142.
Ding W.Q., Lind S.E. Phospholipid hydroperoxide glutathione peroxidase plays a role in protecting cancer cells from docosahexaenoic acid-induced cytotoxicity. Mol. Cancer Ther. 2007;6:1467–1474. doi: 10.1158/1535-7163.MCT-06-0608. [PubMed] [Cross Ref]143.
Vibet S., Goupille C., Bougnoux P., Steghens J.P., Goré J., Mahéo K. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic. Biol. Med. 2008;44:1483–1491. doi: 10.1016/j.freeradbiomed.2008.01.009. [PubMed] [Cross Ref]144.
Sturlan S., Baumgartner M., Roth E., Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxidemediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–4997. doi: 10.1182/blood-2002-08-2391. [PubMed] [Cross Ref]145.
Granci V., Cai F., Lecumberri E., Clerc A., Dupertuis Y.M., Pichar C. Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br. J. Nutr. 2013;109:1188–1195. doi: 10.1017/S000711451200308X. [PubMed] [Cross Ref]146.
Hossain Z., Hosokawa M., Takahashi K. Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutrition and Cancer. 2009;61:123–130. doi: 10.1080/01635580802395725. [PubMed] [Cross Ref]147.
Dai J., Shen J., Pan W., Shen S., Das U.N. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013;12:71. doi: 10.1186/1476-511X-12-71. [PMC free article] [PubMed] [Cross Ref]148.
Jing K., Shin S., Jeong S., Kim S., Song K.S., Park J.H., Heo J.Y., Seo K.S., Park S.K., Kweon G.R., et al. Docosahexaenoic acid induces the degradation of HPV E6/E7 oncoproteins by activating the ubiquitin-proteasome system. Cell Death Dis. 2014;5:e1524. doi: 10.1038/cddis.2014.477. [PMC free article] [PubMed] [Cross Ref]149.
Kang K.S., Wang P., Yamabe N., Fukui M., Jay T., Zhu B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE. 2010;5:15 doi: 10.1371/journal.pone.0010296. [PMC free article] [PubMed] [Cross Ref]150.
Zajdel A., Wilczok A., Tarkowski M. Toxic effects of n-3 polyunsaturated fatty acids in human lung A549 cells. Toxicol. Vitro. 2015 doi: 10.1016/j.tiv.2015.09.013. [PubMed] [Cross Ref]151.
Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: A family of bioactive products of ω-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [PMC free article] [PubMed] [Cross Ref]152.
Wang Q., Hu M., Xu H., Yang X. Anti-inflammatory and Pro-resolving effects of n-3 PUFA in Cancers: Structures and Mechanisms. Curr. Top Med. Chem. 2016;16:888–894. doi: 10.2174/1568026615666150827101602. [PubMed] [Cross Ref]153.
Hawcroft G., Loadman P.M., Belluzzi A., Hull M.A. Effect of eicosapentaenoic acid on E-type prostaglandin synthesis and EP4 receptor signaling in human colorectal cancer cells. Neoplasia. 2010;12:618–627. doi: 10.1593/neo.10388. [PMC free article] [PubMed] [Cross Ref]154.
Poorani R., Bhatt A.N., Dwarakanath B.S., Das U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.08.049. [PubMed] [Cross Ref]155.
Karmali R.A., Reichel P., Cohen L.A., Terano T., Hirai A., Tamura Y., Yoshida S. The effects of dietary ω−3 fatty acids on the DU-145 transplantable human prostatic tumor. Anticancer Res. 1987;17:1173–1180. [PubMed]156.
Rose D.P., Cohen L.A. Effects of dietary menhaden oil and retinyl acetate on the growth of DU145 human prostatic adenocarcinoma cells transplanted into athymic nude mice. Carcinogenesis. 1988;9:603–605. doi: 10.1093/carcin/9.4.603. [PubMed] [Cross Ref]157.
Rose D.P., Connolly J.M. Dietary fat and breast cancer metastasis by human tumor xenografts. Breast Cancer Res. Treat. 1997;46:225–237. doi: 10.1023/A:1005971317978. [PubMed] [Cross Ref]158.
Kobayashi N., Barnard R.J., Henning S.M., Elashoff D., Reddy S.T., Cohen P., Leung P., Hong-Gonzalez J., Freedland S.J., Said J., et al. Effect of altering dietary ω-6/ω-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin. Cancer Res. 2006;12:4662–4670. doi: 10.1158/1078-0432.CCR-06-0459. [PMC free article] [PubMed] [Cross Ref]159.
Funahashi H., Satake M., Hasan S., Sawai H., Newman R.A., Reber H.A., Hines O.J., Eibl G. Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas. 2008;36:353–362. doi: 10.1097/MPA.0b013e31815ccc44. [PubMed] [Cross Ref]160.
Narayanan B.A., Narayanan N.K., Desai D., Pittman B., Reddy B.S. Effects of a combination of docosahexaenoic acid and 1,4-phenylene bis(methylene) selenocyanate on cyclooxygenase 2, inducible nitric oxide synthase and β-catenin pathways in colon cancer cells. Carcinogenesis. 2004;25:2443–2449. doi: 10.1093/carcin/bgh252. [PubMed] [Cross Ref]161.
Calviello G., Serini S., Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: Molecular mechanisms involved. Curr. Med. Chem. 2007;14:3059–3069. doi: 10.2174/092986707782793934. [PubMed] [Cross Ref]162.
Sun H., Berquin I.M., Owens R.T., O’Flaherty J.T., Edwards I.J. Peroxisome proliferator-activated receptor γ-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [PMC free article] [PubMed] [Cross Ref]163.
Edwards I.J., Sun H., Hu Y., Berquin I.M., O’Flaherty J.T., Cline J.M., Rudel L.L., Chen Y.Q. In vivo and in vitro regulation of syndecan 1 in prostate cells by n-3 polyunsaturated fatty acids. J. Biol. Chem. 2008;283:18441–18449. doi: 10.1074/jbc.M802107200. [PMC free article] [PubMed] [Cross Ref]164.
O’Flaherty J.T., Hu Y., Wooten R.E., Horita D.A., Samuel M.P., Thomas M.J., Sun H., Edwards I.J. 15-lipoxygenase metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and survival. PLoS ONE. 2012;7:15 doi: 10.1371/journal.pone.0045480. [PMC free article] [PubMed] [Cross Ref]165.
Hu Y., Sun H., O’Flaherty J.T., Edwards I.J. 15-Lipoxygenase-1-mediated metabolism of docosahexaenoic acid is required for syndecan-1 signaling and apoptosis in prostate cancer cells. Carcinogenesis. 2013;34:176–182. doi: 10.1093/carcin/bgs324. [PMC free article] [PubMed] [Cross Ref]166.
Zou S., Meng X., Meng Y., Liu J., Liu B., Zhang S., Ding W., Wu J., Zhou J. Microarray analysis of anti-cancer effects of docosahexaenoic acid on human colon cancer model in nude mice. Int. J. Clin. Exp. Med. 2015;8:5075–5084. [PMC free article] [PubMed]167.
Sheng H., Li P., Chen X., Liu B., Zhu Z., Cao W. ω-3 PUFAs induce apoptosis of gastric cancer cells via ADORA1. Front. Biosci. 2014;19:854–861. doi: 10.2741/4252. [PubMed] [Cross Ref]
Pooled data showed a reduced incidence of peripheral neuropathy and a preservation of sensory nerve action potential amplitudes with omega-3 supplementation compared with placebo
Omega-3 polyunsaturated fatty acid oral supplements for improving peripheral nerve health: a systematic review and meta-analysis
Published:
17 September 2019
Abstract
Context
Peripheral nerve damage can occur in a variety of systemic conditions and can have a profound impact on functional and psychological health. Currently, therapeutic interventions for peripheral nerve damage are limited.
Objective
The aim of this systematic review, conducted in accordance with the Cochrane Collaboration’s handbook and reported according to the PRISMA checklist, was to evaluate the efficacy and safety of omega-3 oral supplements for improving peripheral nerve structure and function.
Data Sources
PubMed, Embase, and Cochrane databases, along with clinical trial registries, were searched from inception to February 2019. Evidence was identified, critically appraised, and synthesized, and the certainty of evidence was appraised using the Grading of Recommendations Assessment, Development and Evaluation approach.
Study Selection
Randomized controlled trials assessing the effects of omega-3 oral supplementation on outcomes of peripheral nerve structure, peripheral nerve function, or both were eligible for inclusion. Titles and abstracts of identified articles were independently assessed for potential eligibility by 2 review authors. For studies judged as eligible or potentially eligible, full text articles were retrieved and independently assessed by 2 review authors to determine eligibility; disagreements were resolved by consensus.
Data Extraction
Fifteen trials were included. Two clinically similar studies that investigated the effect of omega-3 supplementation in individuals receiving chemotherapy were meta-analyzed. Pooled data showed a reduced incidence of peripheral neuropathy (RR = 0.58; 95%CI, 0.43–0.77) and a preservation of sensory nerve action potential amplitudes with omega-3 supplementation compared with placebo (MD = 4.19 µV; 95%CI; 2.19–6.19).
Conclusion
This review finds, with low certainty, that omega-3 supplementation attenuates sensory loss and reduces the incidence of neuropathy secondary to oxaliplatin and paclitaxel treatment relative to placebo. There is currently limited evidence to ascertain whether omega-3 supplementation is beneficial in other systemic conditions characterized by peripheral nerve damage.
Systematic Review Registration
PROSPERO registration number CRD 42018086297
visolie, EPA, DHA, darmkanker, ziektevrije tijd, progressievrije tijd, Voest, Valstar, voeding, complementair
Gerelateerde artikelen




Plaats een reactie ...
Reageer op "Visolie aanvullend op chemo bij uitgezaaide darmkanker verlengt progressievrije tijd van 11 maanden naar 20 maanden"