lees ook het verhaal van Chris Donaldson die in 2024 werd behandeld met histotripsy voor zijn leveruitzaaiingen afkomstig vanuit zijn oogmelanoom, met uitleg via video hoe histotripsy wordt toegepast
New treatment called histotripsy kills cancer cells with sound and water
In het Providence Mission Hospital kun je via een formulier vragen of je in aanmerking kan komen voor een behandeling met histotripsy.Zie verder ook hieronder meer over histotripsy, over lopende en afgesloten studies enz.
Update 26 mei 2025:
Naar aanleiding van mijn bezoek aan Weber Medical samen met mevrouw R. (uitgezaaide zwaar voorbehandelde borstkanker) ben ik nog eens op zoek gegaan of er nieuwe ontwikkelingen zijn met histotripsy. Op een website van de producent van de histotripsy, een machine die via Ultra Sound kwaadaardige en goedaardige tumoren kan vernietigen zonder gezond tumorweefsel te beschadigen, staan een aantal abstracten van dierstudies, patiëntenstudies en lopende studies in zowel Amerika als in Europa.
Een klein stukje vertaald in het Nederlands uit het Engels, referentienummers staan onderaan in referentielijst:
Onze groep heeft histotripsie bestudeerd voor de behandeling van leverkanker[1,2], prostaatkancer [3-5], nierkanker [6,7], melanomen [8], alvleesklierkanker en immuunstimulatie [8,9]. Ons onderzoek heeft geleid tot een fase I-studie naar de behandeling van goedaardige prostaathyperplasie (NCT01896973), een fase I studie naar de behandeling van leverkanker (NCT03741088) [11,12], en klinische studies in meedere ziekenhuizen naar de behandeling van leverkanker in de VS (NCT04572633) en Europa (NCT04573881), allemaal met behulp van HistoSonics-apparaten.
Onderaan dit artikel staat een referentielijst behorend bij bovenstaande informatie.
Een andere interessante studie is deze, abstract staat v erderop in dit artikel:
Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers
En lees vooral ook verder in dit artikel.......
23 juli 2024: met dank aan Pascale die me dit doorstuurde.
Emma van Dijk, Arts-onderzoeker, internist-oncoloog i.o. laat aan Pascale weten dat de iFocus studie goedkeuring heeft gekregen om patiënten te werven voor een studie waarin histotripsy gecombineerd gaat worden met immuuntherapie met checkpointremmers nivolumab en ipilimumab.
Wie is geïnteresseert lees eerst deze PDF die de studie beschrijft met inclusie- en exclusiecriteria, dus welke kankerpatiënten er wel en niet voor in aanmerking komen.
De fase I studie begint met 6 deelnemers en uiteindelijk zullen aan deze studie 24 personen kunnen deelnemen.
Een belangrijke zin uit de studieopzet: "Histotripsie heeft dus de potentie om een 'koude' niet-immunogene tumor om te zetten in een 'hete' immuunresponsieve tumor"
Zie verder in dit artikel meer informatie over histotripsie.
12 april 2024: Voor wie is geinteresseerd in Ultra Sound behandeling van levertumoren lees dit artikel. Met onderaan in artikel contactadres van arts Matteo Fusaglia die in het Antoni van Leeuwenhoek ziekenhuis voor het NKI de studie met Ultra Sound bij Intra-operatieve navigatie gaat uitvoeren voor zijn promotieonderzoek.
30 november 2023:
Zie ook dit artikel: https://kanker-actueel.nl/histotripsy-een-vorm-van-ultra-sound-met-gepulseerde-geluidsgolven-gericht-op-gasbellen-in-tumorweefsel-is-ook-succesvol-bij-osteosarcomen-in-dierstudies.html
28 november 2023: Zie ook in gerelateerde artikelen
27 november 2023: Bron: Histosonics, 2022;39(1):1115-1123 Met dank aan Jacques die mij deze specifieke Ultra sound behandeling toestuurde.
Ultra Sound is een al tientallen jaren gebruikte techniek om tumorweefsel te vernietigen. Jacques wees me op een hele speciale vorm van Ultra Sound die Histotripsy genoemd.
Histotripsy maakt gebruik van gepulseerde geluidsgolven om “bellenwolken” te veroorzaken uit gassen die van nature aanwezig zijn in het beoogde tumorweefsel. Deze bellenwolken vormen en storten in microseconden in wanneer een arts / PDT specialist het Edison apparaat op de tumoren richt, waardoor mechanische krachten ontstaan die sterk genoeg zijn om weefsel op cellulair en subcellulair niveau te vernietigen op een niet-invasieve en niet-thermische methode.
Histotripsy maakt gebruik van de kracht van microbellen gas om gericht kwaadaardige weefsels te vernietigen en vermijdt ioniserende energie van straling, hitteschade door thermische modaliteiten en gebruikt geen operatie of zelfs geen naalden die traditionele behandelingen wel gebruiken.
Voordelen van de Histotripsy behandeling zijn:
- Het is een veilige en niet invasieve manier van behandelen
- Er is geen operatie of injectie nodig. Alles gaat vanaf de buitenkant en de geluidsgolven (Ultra Sound) doen het werk
- De behandeling kan vele malen herhaald worden
- Er wordt geen normaal celweefsel geraakt en een behandeling geeft daardoor amper bijwerkingen
Nadelen:
- Per behandeling kunnen alleen kleine tumoren in zacht weefsel worden behandeld. Botuitzaaiingen kunnen overigens ook worden behandeld maar zijn alleen nog in dierstudies onderzocht.
- Alleen voor levertumoren is toestemming terwijl deze behandelingen ook voor meerdere vormen van kanker geschikt is
- Alleen een in PDT en Ultra Sound geschoolde arts mag het uitvoeren
Uit een studieverslag:
Hier twee foto's van levertumor, bovenste foto is van voor de behandelingen en daaronder twee maanden later:
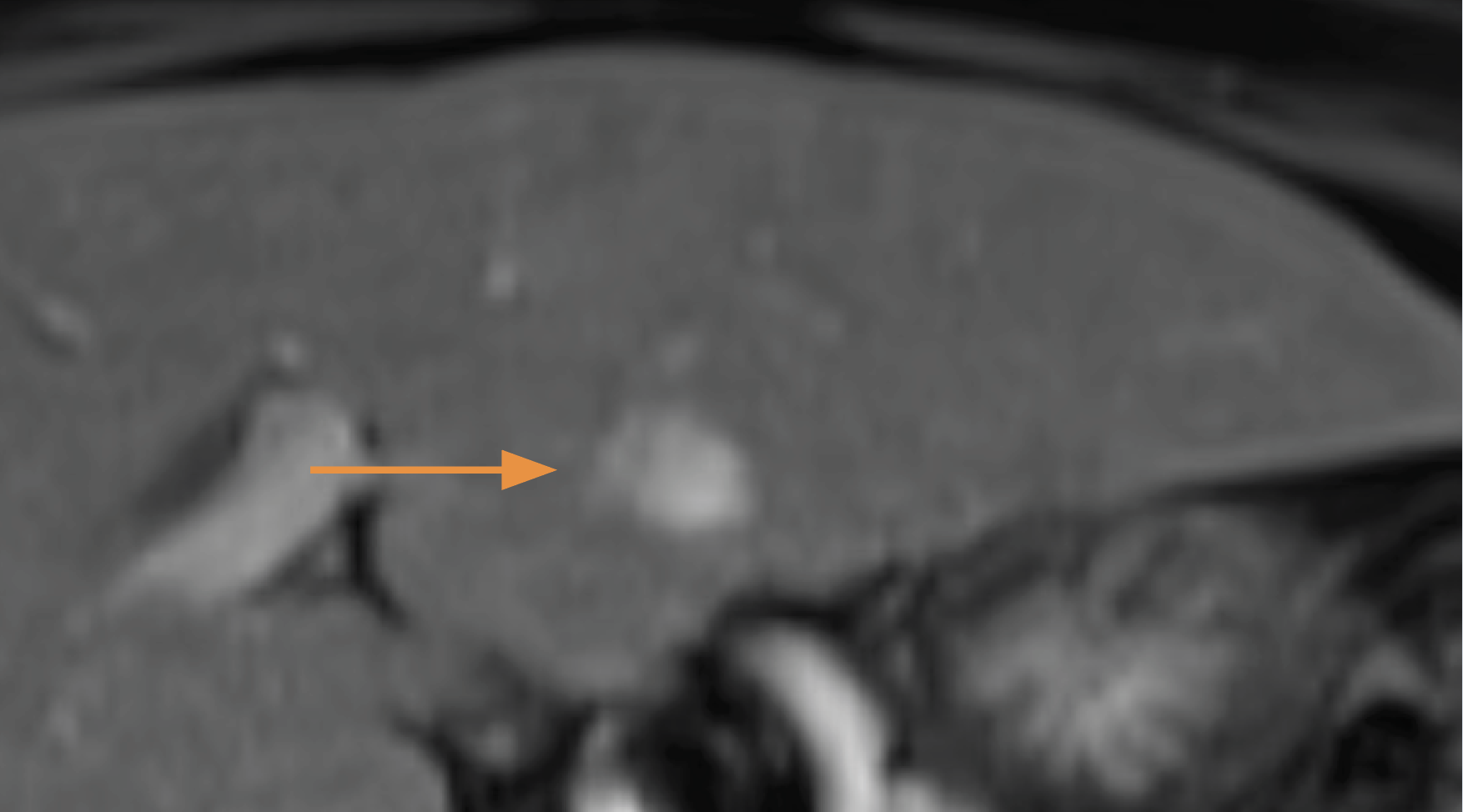
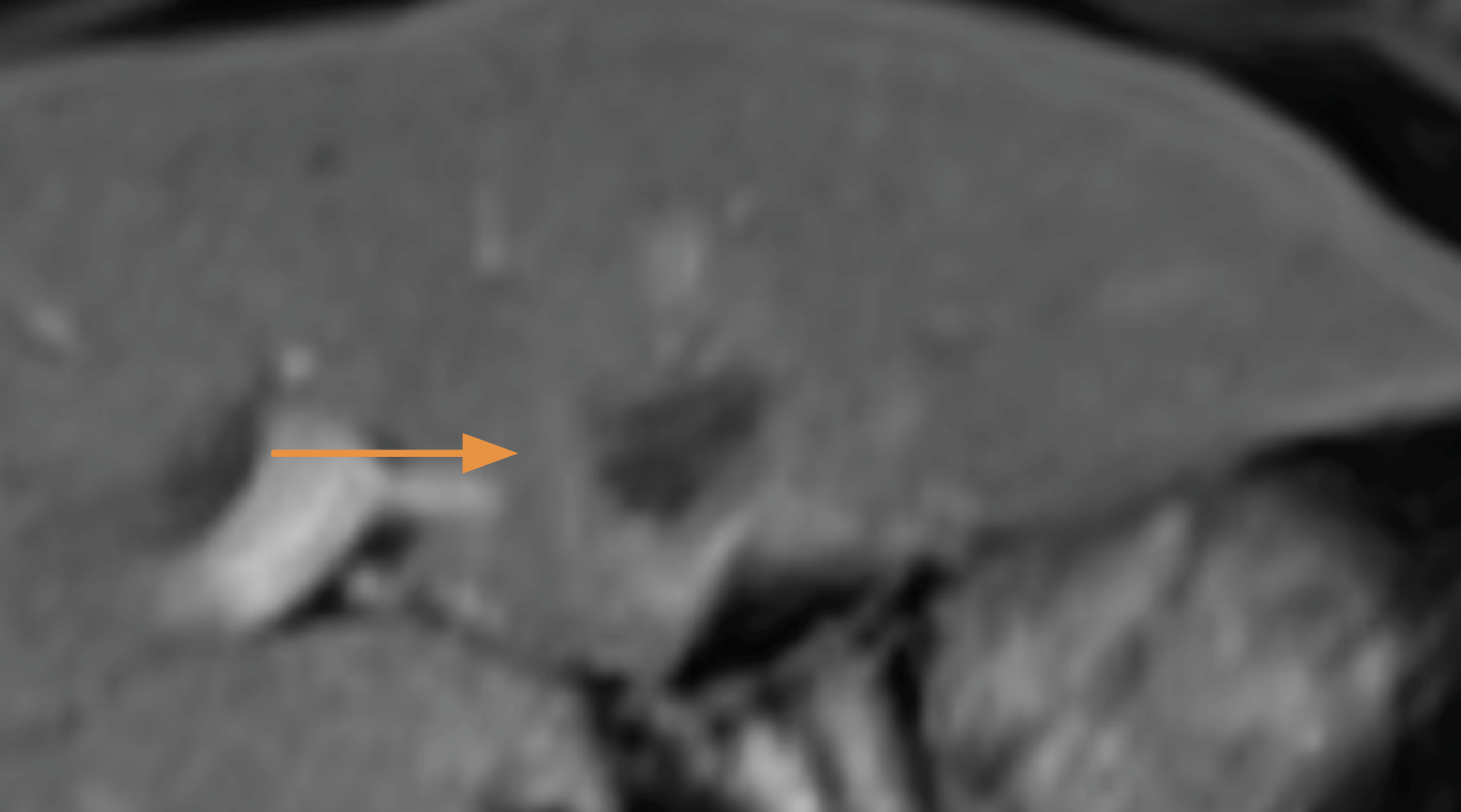
De Amerikaanse FDA keurde al in 2018 de Histotripsy goed om levertumoren niet-invasief te behandelen Gepubliceerd: 9 oktober 2023: https://www.fusfoundation.org/posts/histosonics-focused-ultrasound-platform-receives-fda-breakthrough-device-designation/
HistoSonics – genaamd EdisonTM – goedgekeurd voor de behandeling van levertumoren. Dit is de negende klinische indicatie waarvoor gerichte ultrasone behandeling is goedgekeurd door de FDA.*
EdisonTM maakt gebruik van histotripsy om op niet-invasieve wijze weefsel in de lever te vernietigen en is het eerste en enige platform dat beschikbaar is in de VS en inmiddels ook in Europa. Dit is ook de eerste keer dat het gebruik van histotripsy wereldwijd goedkeuring van de regelgevende instanties heeft gekregen.

De FDA-goedkeuring werd verleend op basis van gegevens uit de klinische onderzoeken #HOPE4LIVER van HistoSonics, waarin deze methode werd getest bij 44 patiënten met primaire en gemetastaseerde levertumoren op 14 locaties in de VS en Europa.
Op deze pagina staan enkele video's over ontstaan van de Histotripsy en verwijzingen naar studies.
Op deze pagina staat het studieprotocol van een lopende studie met referentielijst. In deze studie worden ook loacaties in Europa genoemd waar patienten een behandeling met Histotripsy kunnen krijgen. Artsen kunnen scholing krijgen om met de Histosonic te werken met patiënten: https://histosonics.com/education/
De laatste publicatie is van oktober 2023. Hieronder het abstract met referentielijst maar volledige studierapport is gratis in te zien of te downloaden:
First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study
- PMID: 36002243
- DOI: 10.1080/02656736.2022.2112309
Abstract
Rationale Current hepatic locoregional therapies are limited in terms of effectiveness and toxicities. Given promising pre-clinical results, a first in-human trial was designed to assess the technical effectiveness and safety profile of histotripsy, a noninvasive, non-thermal, non-ionizing focused ultrasound therapy that creates precise, predictable tissue destruction, in patients with primary and secondary liver tumors.
Methods A multicenter phase I trial (Theresa Study) was performed in a single country with 8 weeks of planned follow-up. Eight of fourteen recruited patients were deemed eligible and enrolled in the study. Hepatic histotripsy, was performed with a prototype system (HistoSonics, Inc., Ann Arbor, MI). Eleven tumors were targeted in the 8 patients who all had unresectable end-stage multifocal liver tumors: colorectal liver metastases (CRLM) in 5 patients (7 tumors), breast cancer metastases in 1 (1 tumor), cholangiocarcinoma metastases in 1 (2 tumors), and hepatocellular carcinoma (HCC) in 1 (1 tumor). The primary endpoint was acute technical success, defined as creating a zone of tissue destruction per planned volume assessed by MRI 1-day post-procedure. Safety (device-related adverse events) through 2 months was a secondary endpoint.
Results The 8 patients had a median age of 60.4 years with an average targeted tumor diameter of 1.4 cm. The primary endpoint was achieved in all procedures. The secondary safety profile endpoint identified no device-related adverse events. Two patients experienced a continuous decline in tumor markers during the eight weeks following the procedure.
Conclusions This first-in-human trial demonstrates that hepatic histotripsy effectively destroys liver tissue in a predictable manner, correlating very well with the planned histotripsy volume, and has a high safety profile without any device-related adverse events. Based on these results, the need for more definitive clinical trials is warranted.
Trial Registration: Study to Evaluate VORTX Rx (Theresa). NCT03741088. https://clinicaltrials.gov/ct2/show/NCT03741088 KEY POINTSHistotripsy, a new noninvasive, non-thermal, non-ionizing focused ultrasound therapy, safely created a zone of tissue destruction in the liver that correlated very well with the pre-defined planned tissue destruction volume.In this first human trial histotripsy was well tolerated with no histotripsy device-related adverse events and its primary endpoint of acute technical success was achieved in all 8 enrolled patients with primary or secondary liver tumors.This new locoregional therapy for patients with liver tumors is safe and effective, warranting further trials.
Keywords: Ablation; focused ultrasound; hepatocellular carcinoma (HCC); histotripsy; liver metastasis; therapeutic ultrasound.
Similar articles
-
A Multi-centre, Single Arm, Non-randomized, Prospective European Trial to Evaluate the Safety and Efficacy of the HistoSonics System in the Treatment of Primary and Metastatic Liver Cancers (#HOPE4LIVER).Cardiovasc Intervent Radiol. 2023 Feb;46(2):259-267. doi: 10.1007/s00270-022-03309-6. Epub 2022 Nov 15.PMID: 36380155 Free PMC article.
-
Histotripsy for the Treatment of Cholangiocarcinoma Liver Tumors: In Vivo Feasibility and Ex Vivo Dosimetry Study.IEEE Trans Ultrason Ferroelectr Freq Control. 2021 Sep;68(9):2953-2964. doi: 10.1109/TUFFC.2021.3073563. Epub 2021 Aug 27.PMID: 33856990 Free PMC article.
-
Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model.Ultrasound Med Biol. 2013 Aug;39(8):1398-409. doi: 10.1016/j.ultrasmedbio.2013.02.005. Epub 2013 May 15.PMID: 23683406 Free PMC article.
-
For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy.Ultrasound Med Biol. 2019 May;45(5):1056-1080. doi: 10.1016/j.ultrasmedbio.2018.10.035. Epub 2019 Mar 26.PMID: 30922619 Free PMC article. Review.
-
Mechanical high-intensity focused ultrasound destruction of soft tissue: working mechanisms and physiologic effects.Ultrasound Med Biol. 2015 Jun;41(6):1500-17. doi: 10.1016/j.ultrasmedbio.2015.02.006. Epub 2015 Mar 23.PMID: 25813532 Review.
Cited by
-
Ablative and Immunostimulatory Effects of Histotripsy Ablation in a Murine Osteosarcoma Model.Biomedicines. 2023 Oct 9;11(10):2737. doi: 10.3390/biomedicines11102737.PMID: 37893110 Free PMC article.
-
Impact of immune tolerance mechanisms on the efficacy of immunotherapy in primary and secondary liver cancers.Transl Gastroenterol Hepatol. 2023 Jun 27;8:29. doi: 10.21037/tgh-23-11. eCollection 2023.PMID: 37601739 Free PMC article. Review.
-
Successful In Situ Targeting of Pancreatic Tumors in a Novel Orthotopic Porcine Model Using Histotripsy.Ultrasound Med Biol. 2023 Nov;49(11):2361-2370. doi: 10.1016/j.ultrasmedbio.2023.07.013. Epub 2023 Aug 16.PMID: 37596154 Free PMC article.
-
Magic bubbles: utilizing histotripsy to modulate the tumor microenvironment and improve systemic anti-tumor immune responses.Int J Hyperthermia. 2023;40(1):2244206. doi: 10.1080/02656736.2023.2244206.PMID: 37580047 Review.
-
Bubble Cloud Characteristics and Ablation Efficiency in Dual-Frequency Intrinsic Threshold Histotripsy.ArXiv. 2023 Jul 6:arXiv:2307.03245v1. Preprint.PMID: 37461413 Free PMC article. Updated.
Referenties:
[1] Worlikar, T., Zhang, M., Ganguly, A., Hall, T. L., Shi, J., Zhao, L., Lee, F. T., Mendiratta- Lala, M., Cho, C. S. & Xu, Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers (Basel) 14, 1612, (2022). PMC8996987
[2] Worlikar, T., Mendiratta-Lala, M., Vlaisavljevich, E., Hubbard, R., Shi, J., Hall, T. L., Cho, C. S., Lee, F. T., Greve, J. & Xu, Z. Effects of Histotripsy on Local Tumor Progression in an in vivo Orthotopic Rodent Liver Tumor Model. BME Frontiers, (2020).
[3] Hall, T. L., Hempel, C. R., Wojno, K., Xu, Z., Cain, C. A. & Roberts, W. W. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 74, 932-937., (2009). PMC2757508
[4] Schade, G. R., Styn, N. R., Ives, K. A., Hall, T. L. & Roberts, W. W. Prostate histotripsy: evaluation of prostatic urethral treatment parameters in a canine model. BJU Int 113, 498- 503, (2014). PMC3944657
[5] Schade, G. R., Keller, J., Ives, K., Cheng, X., Rosol, T. J., Keller, E. & Roberts, W. W. Histotripsy Focal Ablation of Implanted Prostate Tumor in an ACE-1 Canine Cancer Model. The Journal of Urology 188, 1957-1964, (2012).
[6] Styn, N. R., Hall, T. L., Fowlkes, J. B., Cain, C. A. & Roberts, W. W. Histotripsy of renal implanted VX-2 tumor in a rabbit model: investigation of metastases. Urology 80, 724- 729, (2012).
[7] Hall, T. L., Kieran, K., Ives, K., Fowlkes, J. B., Cain, C. A. & Roberts, W. W. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. Journal of endourology / Endourological Society 21, 1159-1166, (2007).
[8] Qu, S., Worlikar, T., Felsted, A. E., Ganguly, A., Beems, M. V., Hubbard, R., Pepple, A. L., Kevelin, A. A., Garavaglia, H., Dib, J., Toma, M., Huang, H., Tsung, A., Xu, Z. & Cho, C. S. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 8, (2020). PMC7057529
[9] Pepple, A. L., Guy, J. L., McGinnis, R., Felsted, A. E., Song, B., Hubbard, R., Worlikar, T., Garavaglia, H., Dib, J., Chao, H., Boyle, N., Olszewski, M., Xu, Z., Ganguly, A. & Cho, C. S. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front Immunol 14, 1012799, (2023). PMC9900174
Schuster, T. G., Wei, J. T., Hendlin, K., Jahnke, R. & Roberts, W. W. Histotripsy Treatment of Benign Prostatic Enlargement Using the Vortx Rx System: Initial Human Safety and Efficacy Outcomes. Urology, (2018).
Vidal-Jove, J., Serres, X., Vlaisavljevich, E., Cannata, J., Duryea, A., Miller, R., Merino, X., Velat, M., Kam, Y., Bolduan, R., Amaral, J., Hall, T., Xu, Z., Lee, F. T., Jr. & Ziemlewicz, T. J. First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study. Int. J. Hyperthermia 39, 1115-1123, (2022).
Vidal-Jove, J., Serres-Creixams, X., Ziemlewicz, T. J. & Cannata, J. M. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans Ultrason Ferroelectr Freq Control 68, 3001-3005, (2021).
Vlaisavljevich, E., Kim, Y., Allen, S., Owens, G., Pelletier, S., Cain, C., Ives, K. & Xu, Z. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med. Biol. 39, 1398-1409, (2013). 3709011
Vlaisavljevich, E., Owens, G., Lundt, J., Teofilovic, D., Ives, K., Duryea, A., Bertolina, J., Welling, T. H. & Xu, Z. Non-Invasive Liver Ablation Using Histotripsy: Preclinical Safety Study in an In Vivo Porcine Model. Ultrasound Med. Biol. 43, 1237-1251, (2017).
Kim, Y., Vlaisavljevich, E., Owens, G. E., Allen, S. P., Cain, C. A. & Xu, Z. In vivo transcostal histotripsy therapy without aberration correction. Phys. Med. Biol. 59, 2553- 2568, (2014).
In conclusion, we have qualitatively and quantitatively demonstrated that histotripsy treatment triggers the release of HER2 from the tumor cells into the extracellular compartment. The histotripsy-mediated release of HER2 antigens provides important insights into the mechanism underlying its immunostimulation and suggests the potential of TSA/TAA-based immunotherapies in numerous cancer types.
Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers
Simple Summary
Abstract
Table of Contents
References
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emens, L.A.; Romero, P.J.; Anderson, A.C.; Bruno, T.C.; Capitini, C.M.; Collyar, D.; Gulley, J.L.; Hwu, P.; Posey, A.D., Jr.; Silk, A.W.; et al. Challenges and opportunities in cancer immunotherapy: A Society for Immunotherapy of Cancer (SITC) strategic vision. J. Immunother. Cancer 2024, 12, e009063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vilela, T.; Valente, S.; Correia, J.; Ferreira, F. Advances in immunotherapy for breast cancer and feline mammary carcinoma: From molecular basis to novel therapeutic targets. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189144. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cui, M.; Sun, Y.; Liu, S.; Jiang, W. Mechanisms, combination therapy, and biomarkers in cancer immunotherapy resistance. Cell Commun. Signal. 2024, 22, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, J.W.; Li, Y.; Yang, Y.; Yang, H.K.; Dong, J.M.; Xiao, Z.H.; He, X.; Guo, J.H.; Wang, R.Q.; Dai, B.; et al. Tumor immunotherapy resistance: Revealing the mechanism of PD-1/PD-L1-mediated tumor immune escape. Biomed. Pharmacother. 2024, 171, 116203. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E. Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bader, K.B.; Vlaisavljevich, E.; Maxwell, A.D. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med. Biol. 2019, 45, 1056–1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tumor-Destroying Sound Waves Receive FDA Approval for Liver Treatment in Humans. University of Michigan News. 2023. Available online: https://news.umich.edu/tumor-destroying-sound-waves-receive-fda-approval-for-liver-treatment-in-humans/ (accessed on 20 September 2024).
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pepple, A.L.; Guy, J.L.; McGinnis, R.; Felsted, A.E.; Song, B.; Hubbard, R.; Worlikar, T.; Garavaglia, H.; Dib, J.; Chao, H.; et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front. Immunol. 2023, 14, 1012799. [Google Scholar] [CrossRef]
- Thim, E.A.; Kitelinger, L.E.; Rivera-Escalera, F.; Mathew, A.S.; Elliott, M.R.; Bullock, T.N.J.; Price, R.J. Focused ultrasound ablation of melanoma with boiling histotripsy yields abscopal tumor control and antigen-dependent dendritic cell activation. Theranostics 2024, 14, 1647–1661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hay, A.N.; Vickers, E.R.; Patwardhan, M.; Gannon, J.; Ruger, L.; Allen, I.C.; Vlaisavljevich, E.; Tuohy, J. Investigating cell death responses associated with histotripsy ablation of canine osteosarcoma. Int. J. Hyperth. 2023, 40, 2279027. [Google Scholar] [CrossRef] [PubMed]
- Worlikar, T.; Hall, T.; Zhang, M.; Mendiratta-Lala, M.; Green, M.; Cho, C.S.; Xu, Z. Insights from in vivo preclinical cancer studies with histotripsy. Int. J. Hyperth. 2024, 41, 2297650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, L.; Li, J.; Xu, Q.; Gao, Z.; Yang, M.; Wu, X.; Li, X. HER2/PI3K/AKT pathway in HER2-positive breast cancer: A review. Medicine 2024, 103, e38508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.H.; Lu, L.; Fan, Y.; Wicha, M.S.; Cao, Z.; Chang, A.E.; Xia, J.C.; Baker, J.R., Jr.; Li, Q. Characterization of a novel transgenic mouse tumor model for targeting HER2+ cancer stem cells. Int. J. Biol. Sci. 2013, 10, 25–32. [Google Scholar] [CrossRef] [PubMed Central]
- Chen, J.S.; Chen, J.W.; Bhattacharjee, S.; Cao, Z.; Wang, H.; Swanson, S.D.; Zong, H.; James, R.; Baker, J.R., Jr.; Wang, S.H. Functionalized Nanoparticles with Targeted Antibody to Enhance Imaging of Breast Cancer in vivo. J. Nanobiotechnol. 2020, 18, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidal-Jove, J.; Serres-Creixams, X.; Ziemlewicz, T.J.; Cannata, J.M. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M.; AlJammaz, I. Development of the Tumor-Specific Antigen-Derived Synthetic Peptides as Potential Candidates for Targeting Breast and Other Possible Human Carcinomas. Molecules 2019, 24, 3142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alrhmoun, S.; Sennikov, S. The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions. Cancers 2022, 14, 6173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kellner, C.; Lutz, S.; Oberg, H.H.; Wesch, D.; Otte, A.; Diemer, K.J.; Wilcken, H.; Bauerschlag, D.; Glüer, C.C.; Wichmann, C.; et al. Tumor cell lysis and synergistically enhanced antibody-dependent cell-mediated cytotoxicity by NKG2D engagement with a bispecific immunoligand targeting the HER2 antigen. Biol. Chem. 2021, 403, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, J.S.; Morrison, S.L.; Penichet, M.L. Insights into the mechanism of anti-tumor immunity in mice vaccinated with the human HER2/neu extracellular domain plus anti-HER2/neu IgG3-(IL-2) or anti-HER2/neu IgG3-(GM-CSF) fusion protein. Vaccine 2005, 23, 4793–4803. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zaidi, N.; He, L.Z.; Zhang, L.; Kuroiwa, J.M.; Keler, T.; Steinman, R.M. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012, 14, R39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, F.; Huang, X.; Hong, Z.; Lu, N.; Huang, X.; Liu, L.; Liang, T.; Bai, X. Improvement strategy for immune checkpoint blockade: A focus on the combination with immunogenic cell death inducers. Cancer Lett. 2023, 562, 216167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, G.; Sun, X. Tumor Antigen-Based Nanovaccines for Cancer Immunotherapy: A Review. J. Biomed. Nanotechnol. 2021, 17, 2099–2113. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.T.; Clavijo, P.E.; Van Waes, C.; Chen, Z. Anti-Tumor Immunity in Head and Neck Cancer: Understanding the Evidence, How Tumors Escape and Immunotherapeutic Approaches. Cancers 2015, 7, 2397–2414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le Naour, A.; Koffi, Y.; Diab, M.; Le Guennec, D.; Rougé, S.; Aldekwer, S.; Goncalves-Mendes, N.; Talvas, J.; Farges, M.-C.; Caldefie-Chezet, F.; et al. EO771, the First Luminal B Mammary Cancer Cell Line from C57BL/6 Mice. Cancer Cell Int. 2020, 20, 328. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Luo, Y.; Liu, J.; Wang, X.; Feng, R.; Huang, J.; Du, H.; Li, Q.; Tan, J.; et al. Pharmaceutical Targeting Th2-Mediated Immunity Enhances Immunotherapy Response in Breast Cancer. J. Transl. Med. 2022, 20, 615. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Broggi, G.; Tinnirello, G.; Puzzo, L.; Vecchio, G.M.; Salvatorelli, L.; Memeo, L.; Santoro, A.; Farina, J.; Mulé, A.; et al. Tumor Infiltrating Lymphocytes (TILS) and PD-L1 Expression in Breast Cancer: A Review of Current Evidence and Prognostic Implications from Pathologist’s Perspective. Cancers 2023, 15, 4479. [Google Scholar] [CrossRef]
- Xia, Q.H.; Lu, C.T.; Tong, M.Q.; Yue, M.; Chen, R.; Zhuge, D.L.; Yao, Q.; Xu, H.L.; Zhao, Y.Z. Ganoderma Lucidum Polysaccharides Enhance the Abscopal Effect of Photothermal Therapy in Hepatoma-Bearing Mice Through Immunomodulatory, Anti-Proliferative, Pro-Apoptotic and Anti-Angiogenic. Front. Pharmacol. 2021, 12, 648708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franzese, O.; Torino, F.; Giannetti, E.; Cioccoloni, G.; Aquino, A.; Faraoni, I.; Fuggetta, M.P.; De Vecchis, L.; Giuliani, A.; Kaina, B.; et al. Abscopal Effect and Drug-Induced Xenogenization: A Strategic Alliance in Cancer Treatment? Int. J. Mol. Sci. 2021, 22, 10672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Quan, G.; Wen, T.; Yang, P.; Qin, W.; Mai, H.; Sun, Y.; Lu, C.; Pan, X.; Wu, C. Cold to Hot: Binary Cooperative Microneedle Array-Amplified Photoimmunotherapy for Eliciting Antitumor Immunity and the Abscopal Effect. ACS Appl. Mater. Interfaces 2020, 12, 32259–32269. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; McGinnis, R.; Cao, Z.; Baker, J.R., Jr.; Xu, Z.; Wang, S. Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers. Cancers 2025, 17, 183. https://doi.org/10.3390/cancers17020183
Tang S, McGinnis R, Cao Z, Baker JR Jr., Xu Z, Wang S. Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers. Cancers. 2025; 17(2):183. https://doi.org/10.3390/cancers17020183
Chicago/Turabian StyleTang, Shengzhuang, Reliza McGinnis, Zhengyi Cao, James R. Baker, Jr., Zhen Xu, and Suhe Wang. 2025. "Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers" Cancers 17, no. 2: 183. https://doi.org/10.3390/cancers17020183
APA StyleTang, S., McGinnis, R., Cao, Z., Baker, J. R., Jr., Xu, Z., & Wang, S. (2025). Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers. Cancers, 17(2), 183. https://doi.org/10.3390/cancers17020183
Gerelateerde artikelen
- Histotripsy, een vorm van ultra sound met gepulseerde geluidsgolven en gasbellen blijkt uitstekende, veilige en niet invasieve techniek voor eliminatie van levertumoren, daarbij gezond weefsel sparend
- MRgFUS = MRI-guided focused ultrasound geeft uitstekende resultaten bij bottumoren vanuit verschillende vormen van kanker, maar ook bij goedaardige hersentumoren en essentiele Tremor
- HIFU - High Intensity Focused Ultrasound blijkt een effectieve behandeling voor alvleesklierkanker. HIFU gebruikt gerichte hitte om kankercellen aan te pakken en te vernietigen, en er is minimale schade aan het lichaam.
- UMC Utrecht start studie (i-GO studie ) met chemo via nanodeeltjes plus MR-HIFU voor borstkankerpatienten
- Zelflerende algoritmen leiden tot optimale effectiviteit van door HIFU = Ultra sound opgewekte hyperthermie bij kwaadaardige tumoren, aldus Daniel Deelen in zijn proefschrift
- HIFU - High-Intensity Focused Ultrasound is uitstekende behandeling voor niet uitgezaaide prostaatkanker, waarom blijft dit een experimentele behandeling en wordt HIFU niet vergoed vanuit basisverzekering?
- HIFU - Ultra Sound als pijnbestrijding voor in botten uitgezaaide prostaatkanker in UMC Utrecht
- Ultra sound - HIFU lijkt operatie techniek te worden voor de nabije toekomst. Het MRI-HIFU apparaat is afgelopen jaren met succes gebruikt bij vleesboom verwijdering. Hier meer informatie hoe HIFU - High Focused Ultra Sound precies werkt met foto's enz.
- Ultra Sound - opereren via geluid en verhitting - wordt ingezet bij beginnende borstkanker in het UMC - Utrecht
- Ultra Sound naast TACE geeft hoog significant betere resultaten dan alleen TACE bij levertumoren met een gemiddelde grootte van 10,4 cm. !!!!!, aldus twee gerandomiseerde studies bij totaal 200 patiënten met levertumoren.
- Ultra Sound HIFU - High Dose Focused Ultrasound bij levertumoren en niertumoren blijkt een veilig en met een beter resultaat, significant minder bijwerkingen, uit te voeren behandeling.
- Ultra sound opent tijdelijk blood - brain barriere voor gerichte chemobehandeling van tumoren in het hoofd en hersenen door nanodeeltjes.




Plaats een reactie ...
Reageer op "Histotripsy, een vorm van ultra sound met gepulseerde geluidsgolven en gasbellen blijkt uitstekende, veilige en niet invasieve techniek voor eliminatie van levertumoren, daarbij gezond weefsel sparend"