10 april 2019: Zie ook deze reviewstudie over diagnosetesten en technieken:
19 mei 2017: Bron: American Urological Association (AUA) Annual Meeting 2017
Meer en meer komen er ook voor prostaatkanker aanvullende diagnostische testen zoals urinetesten en genentesten op de markt (zie in gerelateerde artikelen) die kunnen voorspellen of patiënten met een diagnose van operabele prostaatkanker nog verdere behandeling nodig hebben of dat zij kunnen volstaan met een wait-and-see beleid. Aan dit rijtje kan nu ook de GPS (Oncotype DX® Genomic Prostate Score Test) wel worden toegevoegd.
Op de American Urological Association (AUA) Annual Meeting 2017 werden overtuigende resultaten van de efficiëntie van de GPS test gepresenteerd. De GPS test voorspelde in een studie met grote zekerheid hoe groot de kans was dat patiënten met een diagnose van prostaatkanker later uitzaaiingen zouden ontwikkelen en / of zouden overlijden aan hun ziekte. Maar de belangrijkste conclusie was dat voor prostaatkankerpatiënten met een erg laag of laag of gemiddeld risico (Gleasonscore van 7 of minder) die bij de GPS test onder een score van 20 bleven zij verder geen behandeling nodig zouden hebben dan hoogstens een wait-and-see beleid.
(Red: Daarbij opgemerkt dat zover ik dat kan beoordelen geen rekening is gehouden met leefstijl en voedingspatroon van de patiënten dat m.i. in dit verband wel degelijk een rol kan spelen. Want leefstijl en voedingspatroon heeft echt invloed op de progressiviteit van prostaatkanker. Zie ook onze lijst specifiek bij prostaatkanker)
Kernpunten uit de GPS studie:
In deze studie: Health Economic Impact and Prospective Clinical Utility of Oncotype DX® Genomic Prostate Score , gratis in te zien, wordt uitstekend beschreven hoe de GPS test werkt en welke resultaten er uitkomen.
Van deze studie hier het abstract met uitgebreide referentielijst:
Oncotype DX Genomic Prostate Score (GPS) test is a strong independent predictor of prostate cancer-specific death and disease progression at 10 years in men with localized prostate cancer across all clinical risk groups.
Health Economic Impact and Prospective Clinical Utility of Oncotype DX® Genomic Prostate Score.
Abstract
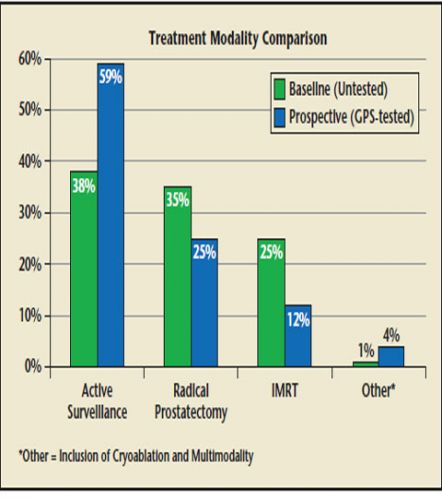
Prostate cancer (CaP) will be diagnosed in approximately 181,000 American men in 2016. Despite the high number of deaths from CaP in the United States, the disease has a protracted natural history and many men diagnosed with CaP will not die of the disease regardless of treatment. Unfortunately, identification of men with truly indolent/ nonaggressive CaP is challenging; limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone. The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit. Incorporation of the Genomic Prostate Score (GPS) as part of the decision algorithm for patients with National Comprehensive Cancer Network very low-risk and low-risk cancer led to a substantial increase in uptake of active surveillance and substantial cost savings. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
- PMID:
- 27833462
- PMCID:
- PMC5102928
- DOI:
- 10.3909/riu0725
-
Conclusions
Incorporation of GPS as part of the decision algorithm for patients with NCCN very-low-risk and lowrisk cancer led to substantial increase in uptake of AS and substantial cost savings (average, $2286 per patient) for insurance carriers. Using the GPS list price of $4520, the $2286 savings represents a return on investment of over 50% ($2286/$4520) over 6 months. Further assessment of GPS in a larger pool of intermediate risk patients is needed to assess the potential impact on treatment planning. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
References
1. SEER Stat Fact Sheets: Prostate Cancer. National Cancer Institute website . http://seer.cancer.gov/statfacts/html/prost.html. Accessed August 20, 2016.2. Xia J, Trock BJ, Cooperberg MR, et al. Prostate cancer mortality following active surveillance versus immediate radical prostatectomy. Clin Cancer Res. 2012;18:5471–5478. [PMC free article] [PubMed]3. Yamamoto T, Musunuru B, Vesprini D, et al. Metastatic prostate cancer in men initially treated with active surveillance. J Urol. 2016;195:1409–1414. [PubMed]4. Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–1024. [PMC free article] [PubMed]5. Wilt TJ, Brawer MK, Jones KM, et al. Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203213. [PMC free article] [PubMed]6. Livingston CJ, Freeman RJ, Mohammad A, et al. Choosing Wisely® Task Force. Choosing Wisely® in Preventive Medicine: the American College of Preventive Medicine’s Top 5 List of Recommendations. Am J Prev Med. 2016;51:141–149. [PubMed]7. Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519–1524. [PubMed]8. Martin NE, Mucci LA, Loda M, Depinho RA. Prognostic determinants in prostate cancer. Cancer J. 2011;17:429–437. [PMC free article] [PubMed]9. Teutsch SM, Bradley LA, Palomaki GE, et al. EGAPP Working Group. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11:3–14. [PMC free article] [PubMed]10. Bostrom PJ, Bjartell AS, Catto JW, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68:1033–1044. [PubMed]11. Cullen J, Rosner IL, Brand TC, et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically lowand intermediate-risk prostate cancer. Eur Urol. 2015;68:123–131. [PubMed]12. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66:550–560. [PubMed]13. Dall’ Era MA, Maddala T, Polychronopoulos L, et al. Utility of the Oncotype DX prostate cancer assay in clinical practice for treatment selection in men newly diagnosed with prostate cancer: a retrospective chart review analysis. Urology Practice. 2015;2:343–348.14. Badani KK, Kemeter MJ, Febbo PG, et al. The impact of a biopsy based 17-gene genomic prostate score on treatment recommendations in men with newly diagnosed clinically prostate cancer who are candidates for active surveillance. Urology Practice. 2015;2:181–189.15. Crawford DE, Gustavsen G, Brawer MB, et al. Evaluation of the economic impact of the CCP assay in localized prostate cancer. Presented at: Society of Urologic Oncology Annual Meeting. 2014. ; December 3-5, ; Bethesda, MD.16. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2016;195:612–618. [PubMed]17. Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics. 2013;14:690. [PMC free article] [PubMed]18. Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314:80–82. [PubMed]19. Vanderlaan BF, Broder MS, Chang EY, et al. Costeffectiveness of 21-gene assay in node-positive, earlystage breast cancer. Am J Manag Care. 2011;17:455–464. [PubMed]20. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. [PMC free article] [PubMed]21. Rizzo JA, Zyczynski TM, Chen J, et al. Lost labor productivity costs of prostate cancer to patients and their spouses: evidence from US National Survey Data. J Occup Environ Med. 2016;58:351–358. [PubMed]22. Tosoian JJ, Loeb S, Epstein JI, et al. Active surveillance of prostate cancer: use, outcomes, imaging, and diagnostic tools. Am Soc Clin Oncol Educ Book. 2016;35:e235–e245. [PMC free article] [PubMed]23. Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304:2373–2380. [PMC free article] [PubMed]24. Johansson E, Steineck G, Holmberg L, et al. SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–899. [PubMed]25. Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. [PubMed]
Articles from Reviews in Urology are provided here courtesy of MedReviews, LLC
Gerelateerde artikelen
- Urinetest met 18 genen ontdekt beter of prostaatkanker hooggradig is of zal worden dan de 2-genentest via PSA blijkt uit nieuwe studie
- Prostaatkanker blijkt zich door genmutaties in twee verschillende vormen te ontwikkelen. Een agressieve vorm en een passieve vorm.
- MRI van prostaat traceert prostaatkanker bij mannen vaker dan een PSA meting en zou sterfte aan prostaatkanker kunnen verminderen
- Nieuwe diagnostische ontwikkelingen (zoals MRI) kunnen agressieve prostaatkanker beter opsporen met minder biopsie procedures en minder overdiagnostiek.
- Combinatie van urinetest op EN2-eiwitmarker en waarden van 10 specifieke genen kan de noodzaak van een biopt aantonen van patiënten met een klinische verdenking van prostaatkanker.
- Oncotype DX Genomic Prostate Score voorspelt nagenoeg zelfde uitkomsten voor blanke en zwarte mannen
- Aanwezigheid van bepaalde mutaties in meting van tumor-DNA via bloedtest na 1 kuur abiraterone voorspelt overlevingskansen van patienten met uitgezaaide prostaatkanker.
- Prostaat-specifiek membraanantigeen PET-CT bij prostaatkankerpatienten voor chirurgie of radiotherapie (proPSMA) geeft betere diagnose dan gewone CT-scan of MRI
- Nieuwe richtlijnen bij diagnostiek van prostaatkanker gaan vandaag in. Eerst MRI daarna pas biopt.
- finasteride kan prostaatkanker voorkomen (min 24 procent). Maar finasteride en dutasteride gebruik veroorzaakt wel bij eerste diagnose hogere Gleasonscores, vaker uitgezaaide ziekte en hogere specifieke sterfte aan prostaatkanker en alle oorzaken
- Effectieve diagnosetechnieken voor prostaatkanker getoetst in reviewstudie. Een prima hulp voor urologen en oncologen hoe prostaatkankerpatienten te behandelen en adviseren.
- Urinetest (SelectMDx) onderscheidt agressiviteit van prostaatkanker en maakt diagnose completer. Radboud ziekenhuis past deze urinetest al toe copy 1
- Toevoeging van het stofje 18F-fluciclovine aan een CT/Pet-scan verandert voorgesteld behandelplan bij 60 procent van de prostaatkankerpatienten
- Bloedtest op circulerende DNA reparatiecellen toont binnen 8 weken aan of een behandeling met olaparib bij prostaatkanker zinvol is.
- Genentest van Oncotype DX (GPS test) voorspelt of prostaatkankerpatienten behandeling nodig hebben of niet na operatie.
- Genenmutaties - 3 groepen - zijn bepalend voor kansen op overall overleving met of zonder bestraling bij prostaatkanker. copy 1
- Bloedtest op aantal, grootte en vorm van circulerende tumorcellen voorspelt welke behandeling beste is bij vergevorderde prostaatkanker: abiraterone en enzalutamide of chemo
- Prolaris, een commerciële genentest voor prostaatkanker, blijkt betrouwbare prognostische informatie te geven op kansen op recidief en verder ziekteverloop van prostaatkanker
- AR-V7 receptor voorspelt welke behandeling zinvol is voor hormoonresistente gevorderde prostaatkanker: chemo of abiraterone en enzalutamide copy 2
- mp-MRI zou beste methode zijn als diagnosetechniek voor vaststellen van prostaatkanker en lijkt superieur aan PSA meting
- BCRA 1 en BCRA 2: prostaatkankerpatienten die drager zijn van het borstkankergen BRCA 1 of BRCA 2 hebben agressievere vorm van prostaatkanker en significant meer kans op overlijden.
- Diagnose prostaatkanker via PSA meting leidt vaak tot onnodige operaties, aldus prof. Schröder.
- Diagnose prostaatkanker: In 30 tot 44 procent van PSA verhoging is sprake van overdiagnose bij mannen met verdenking van prostaatkanker
- DNA testen noodzakelijk voor prostaatkanker met uitzaaingen. Deze heeft meer erfelijk gerelateerde genmutaties dan nog niet uitgezaaide prostaatkanker. copy 1
- Genentest AMACR - naast PSA meting lijkt accurater in vaststellen van prostaatkanker.
- Expressie van eiwit Hsp-27 in urine bij diagnose voorspelt mate van agressiviteit van prostaatkanker en kan wel of niet behandelen bepalen.
- Bloedtest aanvullend op PSA meting geeft significant betere betrouwbaarheid in vaststellen van prostaatkanker.
- Eiwitten S100AS en S100A9 samen met de S100 receptor RAGE lijken bepalend voor diagnose beginnende prostaatkanker
- Mannen met hoog risico op prostaatkanker hebben in voorgaande controles 'normale' PSA waarden. Andere diagnose techieken lijken vereist voor vaststellen van prostaatkanker.
- RECAF - naast PSA meting lijkt accurater in vaststellen van prostaatkanker
- Combidex - Ferumostan-10 geeft betrouwbare diagnostische resultaten (90 tot 100 procent) bij wel of niet uitzaaiingen bij prostaatkanker tot op 2 mm.doorsnede. FDA geeft goedkeuring
- PSA waarden die snel weer stijgen na behandeling zijn voorspeller van slechte prognoses bij prostaatkanker
- Urinetest op PCA3 voorspelt ontwikkeling en verloop van prostaatkanker nauwkeuriger dan PSA test zelf. Ook kan de uitslag van de urinetest PCA3 een biopsie voorkomen en de agressiviteit voorspellen. Gleasonscore blijkt gerelateerd aan de gemeten waarden
- Screening van mannen op prostaatkanker geeft geen verschil in positief effect op succes van eerder behandelen en overall overleving en leidt in tegendeel tot nadelen van overbehandeling blijkt uit groot bevolkingsonderzoek.
- Diagnose: overzicht van artikelen en informatie van diagnose technieken bij verdenking van prostaatkanker



Plaats een reactie ...
Reageer op "Genentest van Oncotype DX (GPS test) voorspelt of prostaatkankerpatienten behandeling nodig hebben of niet na operatie."