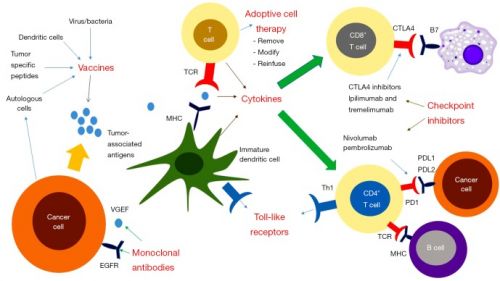
Zie in gerelateerde artikelen voor een overzicht van immuuntherapie waaronder ook dendritische celtherapie bij vormen van darmkanker.
Lees ook de richtlijnen voor het behandelen van uitgezaaide darmkanker zoals gepubliceerd door de ESMO:
Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†
Interessant is ook deze review over zogeheten oncolytische virussen:
Oncolytic viruses: From bench to bedside with a focus on safety
Lees ook dit artikel:
The emerging role of immunotherapy in colorectal cancer
Zie verder in gerelateerde artikelen
Hier een referentielijst van studies met immuuntherapie behorend bij de laatste studievermelding hierboven.:
Referenties:
1. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. 10.3322/caac.21220 [PubMed] [Cross Ref]
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [PubMed] [Cross Ref]
3. Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology 2010;78:237-48. 10.1159/000315730 [PubMed] [Cross Ref]
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. 10.1056/NEJMoa1003466 [PMC free article] [PubMed] [Cross Ref]
5. Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol 2015;6:208-23. [PMC free article] [PubMed]
6. Xiang B, Snook AE, Magee MS, et al. Colorectal cancer immunotherapy. Discov Med 2013;15:301-8. [PMC free article] [PubMed]
7. Koido S, Ohkusa T, Homma S, et al. Immunotherapy for colorectal cancer. World J Gastroenterol 2013;19:8531-42. 10.3748/wjg.v19.i46.8531 [PMC free article] [PubMed] [Cross Ref]
8. Klebanoff CA, Acquavella N, Yu Z, et al. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011;239:27-44. 10.1111/j.1600-065X.2010.00979.x [PMC free article] [PubMed] [Cross Ref]
9. Harris JE, Ryan L, Hoover HC, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000;18:148-57. [PubMed]
10. Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol 2003;21:3343-50. 10.1200/JCO.2003.07.005 [PubMed] [Cross Ref]
11. Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol 2003;21:624-30. 10.1200/JCO.2003.03.091 [PubMed] [Cross Ref]
12. Zheng L, Edil BH, Soares KC, et al. A safety and feasibility study of an allogeneic colon cancer cell vaccine administered with a granulocyte-macrophage colony stimulating factor-producing bystander cell line in patients with metastatic colorectal cancer. Ann Surg Oncol 2014;21:3931-7. 10.1245/s10434-014-3844-x [PMC free article] [PubMed] [Cross Ref]
13. Liebrich W, Schlag P, Manasterski M, et al. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer 1991;27:703-10. 10.1016/0277-5379(91)90170-I [PubMed] [Cross Ref]
14. Schlag P, Manasterski M, Gerneth T, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. First evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother 1992;35:325-30. 10.1007/BF01741145 [PubMed] [Cross Ref]
15. Schulze T, Kemmner W, Weitz J, et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother 2009;58:61-9. 10.1007/s00262-008-0526-1 [PubMed] [Cross Ref]
16. Bartnik A, Nirmal AJ, Yang SY. Peptide Vaccine Therapy in Colorectal Cancer. Vaccines 2012;1:1-16. 10.3390/vaccines1010001 [PMC free article] [PubMed] [Cross Ref]
17. Yamaguchi S, Tatsumi T, Takehara T, et al. EphA2-derived peptide vaccine with amphiphilic poly(gamma-glutamic acid) nanoparticles elicits an anti-tumor effect against mouse liver tumor. Cancer Immunol Immunother 2010;59:759-67. 10.1007/s00262-009-0796-2 [PubMed] [Cross Ref]
18. Moulton HM, Yoshihara PH, Mason DH, et al. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002;8:2044-51. [PubMed]
19. Bilusic M, Heery CR, Arlen PM, et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother 2014;63:225-34. 10.1007/s00262-013-1505-8 [PMC free article] [PubMed] [Cross Ref]
20. Miyagi Y, Imai N, Sasatomi T, et al. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptides. Clin Cancer Res 2001;7:3950-62. [PubMed]
21. Speetjens FM, Kuppen PJK, Welters MJP, et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009;15:1086-95. 10.1158/1078-0432.CCR-08-2227 [PubMed] [Cross Ref]
22. Kimura T, McKolanis JR, Dzubinski LA, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18-26. 10.1158/1940-6207.CAPR-12-0275 [PMC free article] [PubMed] [Cross Ref]
23. Idenoue S, Hirohashi Y, Torigoe T, et al. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 2005;11:1474-82. 10.1158/1078-0432.CCR-03-0817 [PubMed] [Cross Ref]
24. Okuno K, Sugiura F, Hida JI, et al. Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer. Exp Ther Med 2011;2:73-9. [PMC free article] [PubMed]
25. Hazama S, Nakamura Y, Tanaka H, et al. A phase II study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J Transl Med 2014;12:108. 10.1186/1479-5876-12-108 [PMC free article] [PubMed] [Cross Ref]
26. Mayer F, Mayer-Mokler A, Nowara E, et al. A phase I/II trial of the multipeptide cancer vaccine IMA910 in patients with advanced colorectal cancer (CRC). J Clin Oncol 2012;30:abstr 555.
27. Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39:38-48. 10.1016/j.immuni.2013.07.004 [PMC free article] [PubMed] [Cross Ref]
28. Celluzzi CM, Mayordomo JI, Storkus WJ, et al. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med 1996;183:283-7. 10.1084/jem.183.1.283 [PMC free article] [PubMed] [Cross Ref]
29. Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumorlysate-pulsed dendritic cells. Nat Med 1998;4:328-32. 10.1038/nm0398-328 [PubMed] [Cross Ref]
30. Berard F, Blanco P, Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med 2000;192:1535-44. 10.1084/jem.192.11.1535 [PMC free article] [PubMed] [Cross Ref]
31. Koido S, Kashiwaba M, Chen D, et al. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol 2000;165:5713-9. 10.4049/jimmunol.165.10.5713 [PubMed] [Cross Ref]
32. Gong J, Chen D, Kashiwaba M, et al. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 1997;3:558-61. 10.1038/nm0597-558 [PubMed] [Cross Ref]
33. Liu Y, Zhang X, Zhang W, et al. Adenovirus-mediated CD40 ligand gene-engineered dendritic cells elicit enhanced CD8(+) cytotoxic T-cell activation and antitumor immunity. Cancer Gene Ther 2002;9:202-8. 10.1038/sj.cgt.7700429 [PubMed] [Cross Ref]
34. Morse MA, Deng Y, Coleman D, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res 1999;5:1331-8. [PubMed]
35. Fong L, Hou Y, Rivas A, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A 2001;98:8809-14. 10.1073/pnas.141226398 [PMC free article] [PubMed] [Cross Ref]
36. Itoh T, Ueda Y, Kawashima I, et al. Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother 2002;51:99-106. 10.1007/s00262-001-0257-z [PubMed] [Cross Ref]
37. Lesterhuis WJ, de Vries IJM, Schuurhuis DH, et al. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol 2006;17:974-80. 10.1093/annonc/mdl072 [PubMed] [Cross Ref]
38. Maurel J, Caballero-Baños M, Mila J, et al. Phase II randomized trial of autologous tumor lysate dendritic cell vaccine (ADC) plus best supportive care (BSC) compared with BSC, in pre-treated advanced colorectal cancer patients. J Clin Oncol 2015;33:abstr 3048.
39. Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol 2005;23:720-31. 10.1200/JCO.2005.10.206 [PubMed] [Cross Ref]
40. Kaufman HL, Lenz HJ, Marshall J, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res 2008;14:4843-9. 10.1158/1078-0432.CCR-08-0276 [PubMed] [Cross Ref]
41. Chen D, Koido S, Li Y, et al. T cell suppression as a mechanism for tolerance to MUC1 antigen in MUC1 transgenic mice. Breast Cancer Res Treat 2000;60:107-15. 10.1023/A:1006332009414 [PubMed] [Cross Ref]
42. Mukherjee P, Pathangey LB, Bradley JB, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine 2007;25:1607-18. 10.1016/j.vaccine.2006.11.007 [PMC free article] [PubMed] [Cross Ref]
43. Snook AE, Stafford BJ, Li P, et al. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J Natl Cancer Inst 2008;100:950-61. 10.1093/jnci/djn178 [PMC free article] [PubMed] [Cross Ref]
44. Omrane I, Medimegh I, Baroudi O, et al. Involvement of IL17A, IL17F and IL23R Polymorphisms in Colorectal Cancer Therapy. PLoS One 2015;10:e0128911. 10.1371/journal.pone.0128911 [PMC free article] [PubMed] [Cross Ref]
45. Infante JR, Naing A, Papadopoulos KP, et al. A first-in-human dose escalation study of PEGylated recombinant human IL-10 (AM0010) in advanced solid tumors. J Clin Oncol 2015;33:abstr 3017.
46. Wittig B, Schmidt M, Scheithauer W, et al. MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol 2015;94:31-44. 10.1016/j.critrevonc.2014.12.002 [PubMed] [Cross Ref]
47. Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 2008;18:11-8. 10.1016/j.gde.2007.12.007 [PMC free article] [PubMed] [Cross Ref]
48. Thompson MR, Kaminski JJ, Kurt-Jones EA, et al. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011;3:920-40. 10.3390/v3060920 [PMC free article] [PubMed] [Cross Ref]
49. Eiró N, González L, González LO, et al. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol 2012;32:848-54. 10.1007/s10875-012-9666-3 [PubMed] [Cross Ref]
50. Hirsh V, Paz-Ares L, Boyer M, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2667-74. 10.1200/JCO.2010.32.8971 [PubMed] [Cross Ref]
51. Machiels JP, Kaminsky MC, Keller U, et al. Phase Ib trial of the Toll-like receptor 9 agonist IMO-2055 in combination with 5-fluorouracil, cisplatin, and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs 2013;31:1207-16. 10.1007/s10637-013-9933-z [PubMed] [Cross Ref]
52. Weihrauch MR, Richly H, von Bergwelt-Baildon MS, et al. Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. Eur J Cancer 2015;51:146-56. 10.1016/j.ejca.2014.11.002 [PubMed] [Cross Ref]
53. Schmoll HJ, Wittig B, Arnold D, et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol 2014;140:1615-24. 10.1007/s00432-014-1682-7 [PMC free article] [PubMed] [Cross Ref]
54. Riera-Knorrenschild J, Arnold D, Kopp HG, et al. A subgroup with improved overall survival from the phase 2 IMPACT study: Maintenance therapy of metastatic colorectal cancer patients with the TLR-9 agonist MGN1703. J Clin Oncol 2015;33:abstr 3049.
55. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012;12:269-81. 10.1038/nri3191 [PubMed] [Cross Ref]
56. Rosenberg SA, Yang JC, Sherry RM, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin Cancer Res 2011;17:4550-7. 10.1158/1078-0432.CCR-11-0116 [PMC free article] [PubMed] [Cross Ref]
57. Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917-24. 10.1200/JCO.2010.32.2537 [PMC free article] [PubMed] [Cross Ref]
58. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. 10.1056/NEJMoa1215134 [PMC free article] [PubMed] [Cross Ref]
59. Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011;19:620-6. 10.1038/mt.2010.272 [PMC free article] [PubMed] [Cross Ref]
60. Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. 10.1038/mt.2010.24 [PMC free article] [PubMed] [Cross Ref]
61. Zhen YH, Liu XH, Yang Y, et al. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother 2015;64:1083-93. 10.1007/s00262-015-1715-3 [PMC free article] [PubMed] [Cross Ref]
62. Kurniali PC, Hrinczenko B, Al-Janadi A. Management of locally advanced and metastatic colon cancer in elderly patients. World J Gastroenterol 2014;20:1910-22. 10.3748/wjg.v20.i8.1910 [PMC free article] [PubMed] [Cross Ref]
63. Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. 10.1056/NEJMoa0805019 [PubMed] [Cross Ref]
64. Giusti RM, Shastri KA, Cohen MH, et al. FDA drug approval summary: panitumumab (Vectibix). Oncologist 2007;12:577-83. 10.1634/theoncologist.12-5-577 [PubMed] [Cross Ref]
65. Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 2007;12:356-61. 10.1634/theoncologist.12-3-356 [PubMed] [Cross Ref]
66. Wang TF, Lockhart AC. Aflibercept in the treatment of metastatic colorectal cancer. Clin Med Insights Oncol 2012;6:19-30. [PMC free article] [PubMed]
67. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. 10.1016/S0140-6736(12)61900-X [PubMed] [Cross Ref]
68. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565-70. 10.1126/science.1203486 [PubMed] [Cross Ref]
69. Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 2013;19:4917-24. 10.1158/1078-0432.CCR-12-1972 [PMC free article] [PubMed] [Cross Ref]
70. Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. 10.1200/JCO.2010.28.3994 [PubMed] [Cross Ref]
71. Ito A, Kondo S, Tada K, et al. Clinical Development of Immune Checkpoint Inhibitors. Biomed Res Int 2015;2015:605478. [PMC free article] [PubMed]
72. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. 10.1056/NEJMoa1200694 [PMC free article] [PubMed] [Cross Ref]
73. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [PMC free article] [PubMed] [Cross Ref]
74. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. 10.1056/NEJMoa1305133 [PMC free article] [PubMed] [Cross Ref]
75. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. 10.1038/nature13904 [PubMed] [Cross Ref]
76. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [PMC free article] [PubMed] [Cross Ref]
77. Topalian SL, Sznol M, McDermott DF, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol 2014;32:1020-30. 10.1200/JCO.2013.53.0105 [PMC free article] [PubMed] [Cross Ref]
78. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. 10.1056/NEJMoa1500596 [PMC free article] [PubMed] [Cross Ref]
79. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. 10.1056/NEJMoa1302369 [PubMed] [Cross Ref]
80. Teply BA, Lipson EJ. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park) 2014;28:30-8. [PubMed]
81. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014;2:632-42. 10.1158/2326-6066.CIR-14-0053 [PMC free article] [PubMed] [Cross Ref]
Articles from Annals of Translational Medicine are provided here courtesy of AME Publications
Gerelateerde artikelen
- Nous-209, gepersonaliseerde immuungerichte vaccins tonen veelbelovende resultaten bij het voorkomen van erfelijke kanker bij patiënten met het Lynchsyndroom, waaronder blaaskanker, buikvlieskanker, darmkanker, longkanker copy 1
- crispr-cas9-bewerkte T-cellen gericht op CISH maakt patienten met gevorderde darmkanker en GIST alsnog gevoelig voor immuuntherapie met anti-PD medicijnen
- Immuuntherapie met nivolumab plus ipilimumab vooraf aan operatie bij darmkankerpatienten met hoge waarden van microsatellite-instability high/mismatch repair deficient (MSI-H/dMMR) blijkt zeer effectief.
- Immuuntherapie met Pembrolizumab geeft veel betere resultaten op ziektevrije overleving (48 vs 18 procent op 2 jaars meting) dan chemotherapie voor uitgezaaide darmkanker met MSI-H/dMMR - of afwijkende reparatie genen
- KRAS gemuteerde tumoren: Kankervaccin ELI-002 2P stimuleerde hoge T-celreacties bij patiënten met voor immuuntherapie ongevoelige KRAS-gemuteerde tumoren en verbeterde de ziekteprogressieve tijd bij patienten met alvleesklierkanker en darmkanker copy 1 co
- Immuuntherapie vooraf aan operatie en chemotherapie blijkt succesvol bij kankerpatiënten met maagkanker en met tumoren op de overgang van slokdarm naar de maag copy 2
- Dostarlimab, een specifieke vorm van een anti-PD medicijn is 100 procent effectief bij alle 12 patienten met operabele rectumkanker met dMMR = Mismatch-reparatie-deficiëntie en was geen operatie meer nodig.
- Kankerpatienten met solide tumoren met MSI-H = hoge microsatelliet instabiliteit en mismatch reparatie (dMMR) reageren uitstekend op immuuntherapie met pembrolizumab vooraf aan operatie met 65 tot 80 procent complete remissies copy 1
- Immuuntherapie met nivolumab plus ipilimuab voorafgaand aan operatie darmkanker geeft uitstekende resultaten met duurzame klinische complete remissies
- Temozolomide - temodal gevolgd door immunotherapie met combinatie van lage dosis ipilimumab plus nivolumab geeft hoopgevende resultaten bij patiënten met microsatellietstabiel en MGMT-gedempte uitgezaaide darmkanker
- CYAD-101, een vorm van CAR-T cel immuuntherapie geeft hoopvolle resultaten bij uitgezaaide darmkanker zonder dat graft-versus-host-ziekte ontstaat.
- Dendritische cellen en Newcastle Disease Virus bij kankerpatiënten met spijsverteringskanker geeft significant betere resultaten in overlevingstijd aldus gerandomiseerde studie bij 335 patiënten. copy 2
- Autologe genetisch gemodificeerde T-cellen gericht tegen het humaan papillomavirus (HPV) 16 E6 geeft bij patienten met vergevorderde zwaar voorbehandelde uitgezaaide HPV gerelateerde kanker uitstekende resultaten copy 1
- Man met uitgezaaide darmkanker stadium 4 (KRAS pos.) geneest alsnog met combinatie van immuuntherapie met Rigvir virus (dendritische celtherapie) plus FOLFOX en bevacizumab en is nu na 8 jaar kankervrij.
- Nivolumab (Opvido) + ipilimumab (Yervoy) geeft uitstekende resultaten bij nog niet behandelde uitgezaaide darmkanker (met MSI-H of dMMR mutaties) en bereikte 53 procent een gedeeltelijke remissie en 7 procent een complete remissie.
- Immuuntherapie met een gemoduleerd virus plus avelumab, een anti-PD medicijn, wordt onderzocht in een fase I/II studie bij darmkankerpatienten
- Man met uitgezaaide inoperabele darmkanker komt met ATID - Autologous tumor immunizing devascularization, een vorm van immuuntherapie in complete remissie en is al 16 jaar kankervrij, zonder chemo of andere behandelingen
- Immuuntherapie met autovaccinatie van dendritische cellen moet weer eerstelijns behandeling worden voor operabele darmkanker stadium II en III
- Immuuntherapie met dendritische celtherapie geeft uitstekende resultaten op overall overleving en ziektevrije tijd bij darmkanker met weinig of geen zichtbare tumoren.
- Xilonix - MAPp1 zorgt voor stabiele ziekte (bij 53 procent) bij zwaar voorbehandelde darmkankerpatienten stadium 4 met een mediane overall overleving van 4.2 maanden vs 11.5 maanden in vergelijking met placebo copy 1
- Dendritische cellen en Newcastle Disease Virus bij kankerpatiënten met spijsverteringskanker geeft significant betere resultaten in overlevingstijd aldus gerandomiseerde studie bij 335 patiënten. copy 1
- Immuuntherapie: Oncophage(R) (HSPPC-96), gemaakt van eigen tumorcellen zorgt voor opmerkelijke resultaten bij o.a. darmkanker.
- Immuuntherapie met het gemoduleerde virus Ankara–5T4 (TroVax) plus lage dosis cyclophosphamide zorgt voor verdubbeling van mediane overall overleving 11,2 vs 20 maanden bij vergevorderde darmkanker
- Immuuntherapie geeft uitstekende resultaten bij darmkanker met minimale ziekte - weinig tumoren - en zelfs bij darmkanker stadium IV werkt het ook al is het dan minder effectief
- Immuuntherapie met een moleculair middel - codenaam MGN1703 - geeft hoog significant betere ziektevrije tijd bij patiënten met uitgezaaide darmkanker die eerder chemotherapie kregen.
- Autovaccinatie bij darmkanker. Oncovax geeft significant beter resultaat op overleving, overlevingstijd en tijd tot recidief bij darmkankerpatiënten met stadum II. Intracel is failliet verklaard. Oncovax wordt niet meer geleverd
- Archief: immuuntherapie met CEA anti-body plus bestraling geeft hoopvolle resultaten in Phase-II trial met 30 darmkankerpatiënten.
- Regulier - Darmkankers: recente ontwikkelingen en belangrijke studies - artikelen over behandelen en omgaan met vormen van darmkanker binnen de reguliere oncologie: een overzicht