28 maart 2017 Lees ook dit artikel:
Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
29 juni 2015: Bron: J Transl Med. 2011; 9: 17. Published online 2011 Jan 27. doi: 10.1186/1479-5876-9-17
Immuuntherapie geeft bij darmkanker met minimale ziekte - weinig zichtbare tumoren - uitstekende resultaten op ziektevrije tijd, kans op recidief en overall overleving. Dit geldt voor zowel darmkanker stadium I, II, en III waarbij debulking of andere behandeling die tumoren in aantal en omvang deed afnemen werd toegepast. In vergelijking met controlegroepen die geen immuuntherapie werd aangeboden waren de verschillen statistisch significant. Voor gevorderde damkanker stadium IV was er een iets beter resultaat maar veel minder dan voor de andere stadia met minimale ziekte. Dit blijkt uit een grote review studie van totaal 49 studies uitgevoerd in een periode van 12 jaar (1998 t/m 2010). Algemeen blijkt het overall resultaat nog altijd tussen de 22% en 31% te bedragen in gevorderde darmkanker, zie deze grafiek
Bovenstaande zijn de meest gebruikte vormen van immuuntherapie
Dat immuuntherapie het beste werkt bij minimale ziekte, weinig tumoren in het lichaam, of na debulking operatie of na eerdere andere behandelingen of al in beginstadium van de ziekte is in feite algemeen bekend. Maar studies worden nog bijna altijd alleen gedaan bij mensen met vergevorderde ziekte na falen van vele andere behandelingen. En waarbij dan weer DNA mutaties ontstaan die minder gevoelig lijken voor immuuntherapie. En niet opbelangrijk: daarvoor dan hele dure personalised medicine worden ingezet zonder debulking vooraf.
Ik weet dat voor darmkankerpatienten die succesvol geopereerd zijn zelden of nooit immuuntherapie, met bv. autovaccinatie, zie in gerelateerde artikelen, wordt aangeboden, terwijl studies hebben uitgewezen dat autovaccinatie superieure resultaten laat zien in vergelijking met eerstelijns standaard behandelingen na operatie met chemo combinaties. En nog een groot voordeel, immuuntherapie laat zelden enstige bijwerkingen zien, zeker niet bij minimale ziekte.
Bij patiënten met darmkanker in stadium II en III met, al of neit debulking, een minimale ziekte verdubbelde de ziektevrije tijd met ASI, zie deze grafiek.
Zo laat nu dus ook deze reviewstudie in alle resultaten zien dat ASI - actieve specifieke immuuntherapie in feite eerste keus zou moeten zijn, zeker bij darmkanker met minimale ziekte:
Resultaten:
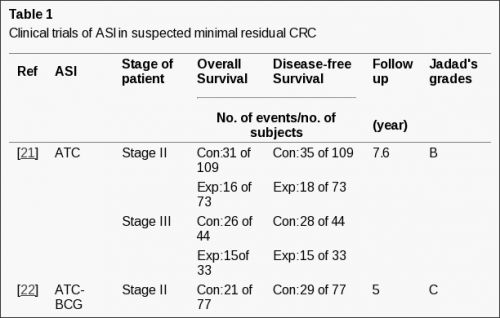
1375 patiënten met vormen van darmkanker, waaronder ook endeldarmkanker, met minimale ziekte (weinig tot geen zichtbare tumoren) werden geëvalueerd in een meta-analyse:
- een algemeen statistisch significante overall overleving en ziektevrije tijd werd gevonden voor ASI - actieve specifieke vorm van immuuntherapie (voor OS - overall overleving: HR = 0.76, P = 0.007; voor DFS - ziektevrije tijd: HR = 0.76, P = 0.03).
- Voor ASI in stadium II met veronderstelde minimale ziekte, OS bereikte statistische significante verschillen met de controlegroepen (HR = 0.71, P = 0.09);
- het verschil in ziektevrije tijd voor ASI voor stadium II met veronderstelde minimale ziekte bereikte statistische significantie (HR = 0.66, P = 0.02).
- Voor ASI bij darmkanker stadium III met na bv. debulking veronderstelde minimale ziekte bereikte statistische significantie voor zowel OS en DFS - ziektevrije tijd (Voor OS: HR = 0.76, P = 0.02; Voor DFS: HR = 0.81, P = 0.03).
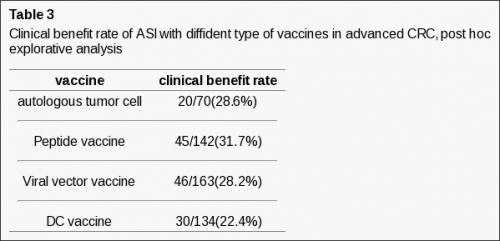
- 656 patiënten met vergevorderde darmkanker stadium werden ook geanalyseerd in een meta-analyse. 11 complete remissies en partiële remissies werden gezien wat betekent een overall gemeten response van 1,68%.
- Er werden overall bij alle 2031 deelnemende patiënten geen ernstige bijwerkingen gemeld gerelateerd aan de vormen van immuuntherapie die waren toegepast.
Conclusies:
De onderzoekers stellen dan ook dat ASI - actieve specifieke vorm van immuuntherapie waarschijnlijk geen standaardbehandeling kan worden voor darmkanker in stadium IV met vergevorderde ziekte. Echter de resultaten geven ook aan dat ASI - actieve specifieke vorm van immuuntherapie voor darmkanker in stadia I, II, en III hoopgevend zijn en het is duidelijk dat immuuntherapie het beste werkt bij darmkankerpatiënten met minimale ziekte, weinig tot geen zichtbare tumoren.
Reden te meer om dit rapport eens mee te nemen naar uw behandelend arts of hem/haar toe te sturen. Zefls als u op dit moment veel uitzaaiingen hebt. Met debulking technieken kan tegenwoordig aardig wat worden weggehaald en misschien heeft dan een ASI - actieve specifieke vorm van immuuntherapie nog steeds zin? Voor mensen met nieuwe diagnose van bv. operabele darmkanker en nog weinig zichtbare tumoren of aantoonbaar geen uitzaaiingen heeft een gesprek hierover zeker zin lijkt mij.
In het studierapport: Clinical outcomes of active specific immunotherapy in advanced colorectal cancer and suspected minimal residual colorectal cancer: a meta-analysis and system review dat gratis is in te zien staan heel veel meer gegevens met mooie grafieken enz.
Hier het abstract van de studie.
This Meta-analysis and System Review clearly supports the idea that a statistically significantly improved DFS or OS was shown in all stage suspected minimal residual CRC patients. Meanwhile, there was also a clear indication that the objective clinical outcome of ASI in advanced CRC was only 1.6%. The results showed it is unlikely that ASI will provide a standard complementary therapeutic approach for advanced CRC in the near future. However, it has become clear that immunotherapy works best in situations of patients with suspected minimal residual CRC
Clinical outcomes of active specific immunotherapy in advanced colorectal cancer and suspected minimal residual colorectal cancer: a meta-analysis and system review
Abstract
Background
To evaluate the objective clinical outcomes of active specific immunotherapy (ASI) in advanced colorectal cancer (advanced CRC) and suspected minimal residual colorectal cancer (suspected minimal residual CRC).
Methods
A search was conducted on Medline and Pub Med from January 1998 to January 2010 for original studies on ASI in colorectal cancer (CRC). All articles included in this study were assessed with the application of predetermined selection criteria and were divided into two groups: ASI in advanced CRC and ASI in suspected minimal residual CRC. For ASI in suspected minimal residual CRC, a meta-analysis was executed with results regarding the overall survival (OS) and disease-free survival (DFS). Regarding ASI in advanced colorectal cancer, a system review was performed with clinical outcomes.
Results
1375 colorectal carcinoma patients with minimal residual disease have been enrolled in Meta-analysis. A significantly improved OS and DFS was noted for suspected minimal residual CRC patients utilizing ASI (For OS: HR = 0.76, P = 0.007; For DFS: HR = 0.76, P = 0.03). For ASI in stage II suspected minimal residual CRC, OS approached significance when compared with control (HR = 0.71, P = 0.09); however, the difference in DFS of ASI for the stage II suspected minimal residual CRC reached statistical significance (HR = 0.66, P = 0.02). For ASI in stage III suspected minimal residual CRC compared with control, The difference in both OS and DFS achieved statistical significance (For OS: HR = 0.76, P = 0.02; For DFS: HR = 0.81, P = 0.03). 656 advanced colorectal patients have been evaluated on ASI in advanced CRC. Eleven for CRs and PRs was reported, corresponding to an overall response rate of 1.68%. No serious adverse events have been observed in 2031 patients.
Conclusions
It is unlikely that ASI will provide a standard complementary therapeutic approach for advanced CRC in the near future. However, the clinical responses to ASI in patients with suspected minimal residual CRC have been encouraging, and it has become clear that immunotherapy works best in situations of patients with suspected minimal residual CRC.
Referentielijst:
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [PubMed] [Cross Ref]
- Kerr D. Clinical development of gene therapy for colorectal cancer. Nat Rev Cancer. 2003;3:615–622. doi: 10.1038/nrc1147. [PubMed] [Cross Ref]
- Lorenz M, Staib-Sebler E, Hochmuth K, Heinrich S, Gog C, Vetter G, Encke A, Muller HH. Surgical resection of liver metastases of colorectal carcinoma: short and long-term results. Semin Oncol. 2000;27:112–119. [PubMed]
- Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [PubMed] [Cross Ref]
- Tebbutt NC, Cattell E, Midgley R, Cunningham D, Kerr D. Systemic treatment of colorectal cancer. Eur J Cancer. 2002;38:1000–1015. doi: 10.1016/S0959-8049(02)00062-X. [PubMed] [Cross Ref]
- SEER Cancer Statistics Review, 1975-2003. National Cancer Institute, retrieved; 2006. http://seer.cancer.gov/csr/1975-2003/
- Smith RE, Colangelo L, Wieand HS, Begovic M, Wolmark N. Randomized trial of adjuvant therapy in colon carcinoma: 10-year results of NSABP protocol C-01. J Natl Cancer Inst. 2004;96:1128–1132. doi: 10.1093/jnci/djh220. [PubMed] [Cross Ref]
- Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S, Ishikawa H, Kawate S, Nakajima T, Morishita Y. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. Br J Cancer. 2004;90:1003–1010. doi: 10.1038/sj.bjc.6601619. [PMC free article] [PubMed] [Cross Ref]
- Wolmark N, Bryant J, Smith R, Grem J, Allegra C, Hyams D, Atkins J, Dimitrov N, Oishi R, Prager D, Fehrenbacher L, Romond E, Colangelo L, Fisher B. Adjuvant 5-uorouracil and leucovorin with or without interferon alfa-2a in colon carcinoma: National Surgical Adjuvant Breast and Bowel Project protocol C-05. J Natl Cancer Inst. 1998;90:1810–1816. doi: 10.1093/jnci/90.23.1810. [PubMed] [Cross Ref]
- Morse M, Langer L, Starodub A, Hobeika A, Clay T, Lyerly HK. Current Immunotherapeutic Strategies in Colon Cancer. Surg Oncol Clin N Am. 2007;16:873–900. doi: 10.1016/j.soc.2007.07.005. [PubMed] [Cross Ref]
- Mocellin S, Mandruzzato S, Bronte V, Marincola FM. Cancer vaccines: pessimism in check. Nat Med. 2004;10:1278–1279. doi: 10.1038/nm1204-1278. [PubMed] [Cross Ref]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [PMC free article] [PubMed] [Cross Ref]
- Nagorsen D, Thiel E. Clinical and Immunologic Responses to Active Specific Cancer Vaccines in Human Colorectal Cancer. Clin Cancer Res. 2006;12:3064–3069. doi: 10.1158/1078-0432.CCR-05-2788. [PubMed] [Cross Ref]
- Alejandro R, Jadad MD, DPhil R, Andrew Moore DPhil, Dawn Carroll RGN, Crispin Jenkinson DPhil D, John M, Reynolds DPhil, David J, Gavaghan DPhil, Henry J, McQuay DM. Assessing the quality of reports of randomized clinical t rials: Is blinding necessary ? Control Clinic Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [Cross Ref]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [PubMed] [Cross Ref]
- Harris JE, Ryan L, Hoover HC Jr, Stuart RK, Oken MM, Benson AB, Mansour E, Haller DG, Manola J, Hanna MG Jr. Adjuvant Active Specific Immunotherapy for Stage II and III Colon Cancer With an Autologous Tumor Cell Vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol. 2000;18:148–153. [PubMed]
- Uyl-de Groot CA, Vermorken JB, Hanna MG Jr, Verboom P, Groot MT, Bonsel GJ, Meijer CJ, Pinedo HM. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benets. Vaccine. 2005;23:2379–2387. doi: 10.1016/j.vaccine.2005.01.015. [PubMed] [Cross Ref]
- Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58:61–69. doi: 10.1007/s00262-008-0526-1. [PubMed] [Cross Ref]
- Riethmüller G, Holz E, Schlimok G, Schmiegel W, Raab R, Höffken K, Gruber R, Funke I, Pichlmaier H, Hirche H, Buggisch P, Witte J, Pichlmayr R. Monoclonal Antibody Therapy for Resected Dukes' Colorectal Cancer:Seven-Year Outcome of a Multicenter Randomized Trial. J Clin Oncol. 1998;16:1788–1794. [PubMed]
- Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y, Li CL, Meng FJ. et al. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive traet. World J Gastroenterol. 2003;9:495–498. [PubMed]
- Tarasov VA, Filatov MV, Kisliakova TV, Noskov FS, Koloskov AV, Stavrovietski VV, Onikienko SB, Kletchikov VZ, Lvov IV, Yu Varfolomeeva E, Blizniukov OP, Levina VV, Kiselevski MV. Combined Surgical and Immunotherapeutic Treatment of Patients with Fourth Stage Colon Cancer. Hybridoma. 1999;18:99–102. doi: 10.1089/hyb.1999.18.99. [PubMed] [Cross Ref]
- Bhattachary-Chatterjee M, Nath Baral R, Chatterjee SK, Das R, Zeytin H, Chakraborty M, Foon KA. Counterpoint. Cancer vaccines: single-epitope anti-idiotype vaccine versus multiple- epitope antigen vaccine. Cancer Immunol Immunother. 2000;49:133–141. doi: 10.1007/s002620050612. [PubMed] [Cross Ref]
- Conry RM, Curiel DT, Strong TV, Moore SE, Allen KO, Barlow DL, Shaw DR, LoBuglio AF. Safety and Immunogenicity of a DNA Vaccine Encoding Carcinoembryonic Antigen and Hepatitis B Surface Antigen in Colorectal Carcinoma Patients. Clin Cancer Res. 2002;8:2782–2787. [PubMed]
- Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [PMC free article] [PubMed] [Cross Ref]
- Hörig H, Lee DS, Conkright W, Divito J, Hasson H, LaMare M, Rivera A, Park D, Tine J, Guito K, Tsang KW, Schlom J, Kaufman HL. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49:504–514. [PubMed]
- Itoh T, Ueda Y, Kawashima I, Nukaya I, Fujiwara H, Fuji N, Yamashita T, Yoshimura T, Okugawa K, Iwasaki T, Ideno M, Takesako K, Mitsuhashi M, Orita K, Yamagishi H. Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother. 2002;51:99–106. doi: 10.1007/s00262-001-0257-z. [PubMed] [Cross Ref]
- Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y, Li CL, Meng FJ. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol. 2003;9:495–498. [PubMed]
- Liu KJ, Wang CC, Chen LT, Cheng AL, Lin DT, Wu YC, Yu WL, Hung YM, Yang HY, Juang SH, Whang-Peng J. Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res. 2004;10:2645–2651. doi: 10.1158/1078-0432.CCR-03-0430. [PubMed] [Cross Ref]
- Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, Tsang KY, Schlom J. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Onco. 2000;18:3964–3973. [PubMed]
- Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Grosenbach DW, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [PubMed] [Cross Ref]
- Matsuda K, Tsunoda T, Tanaka H, Umano Y, Tanimura H, Nukaya I, Takesako K, Yamaue H. Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2004;53:609–616. doi: 10.1007/s00262-003-0491-7. [PubMed] [Cross Ref]
- Miyagi Y, Imai N, Sasatomi T, Yamada A, Mine T, Katagiri K, Nakagawa M, Muto A, Okouchi S, Isomoto H, Shirouzu K, Yamana H, Itoh K. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptides. Clin Cancer Res. 2001;7:3950–3962. [PubMed]
- Morse MA, Nair SK, Mosca PJ, Hobeika AC, Clay TM, Deng Y, Boczkowski D, Proia A, Neidzwiecki D, Clavien PA, Hurwitz HI, Schlom J, Gilboa E, Lyerly HK. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest. 2003;21:341–349. doi: 10.1081/CNV-120018224. [PubMed] [Cross Ref]
- Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, Niedzwiecki D, Panicali D, Schlom J, Lyerly HK. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [PubMed] [Cross Ref]
- Rains N, Cannan RJ, Chen W, Stubbs RS. Development of a dendritic cell (DC)-based vaccine for patients with advanced colorectal cancer. Hepatogastroenterology. 2001;48:347–351. [PubMed]
- Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K, Akiyoshi T, Mori M. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res. 2001;7:2277–2284. [PubMed]
- Sato Y, Maeda Y, Shomura H, Sasatomi T, Takahashi M, Une Y, Kondo M, Shinohara T, Hida N, Katagiri K, Sato K, Sato M, Yamada A, Yamana H, Harada M, Itoh K, Todo S. A phase I trial of cytotoxic T- lymphocyte precursor-oriented peptide vaccines for colorectal carcinoma patients. Br J Cancer. 2004;90:1334–1342. doi: 10.1038/sj.bjc.6601711. [PMC free article] [PubMed] [Cross Ref]
- Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi K, Katsuramaki T, Yasoshima T, Sasaki K, Mizushima Y, Minamida H, Kimura H, Akiyama M, Hirohashi Y, Asanuma H, Tamura Y, Shimozawa K, Sato N, Hirata K. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19–24. doi: 10.1186/1479-5876-2-19. [PMC free article] [PubMed] [Cross Ref]
- Ueda Y, Itoh T, Nukaya I, Kawashima I, Okugawa K, Yano Y, Yamamoto Y, Naitoh K, Shimizu K, Imura K, Fuji N, Fujiwara H, Ochiai T, Itoi H, Sonoyama T, Hagiwara A, Takesako K, Yamagishi H. Dendritic cell-based immunotherapy of cancer with carcinoembryonic antigen-derived, HLA-A24-restricted CTL epitope: clinical outcomes of 18 patients with metastatic gastrointestinal or lung adenocarcinomas. Int J Oncol. 2004;24:909–917. [PubMed]
- von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, Davey M, McLaughlin S, Schlom J, Weiner LM. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6:2219–2228. [PubMed]
- Lasalvia-Prisco E, Garcia-Giralt E, Cucchi S, Vázquez J, Lasalvia-Galante E, Golomar W, Larrañga J. Advanced Colon Cancer: Antiprogressive Immunotherapy Using an Autologous Hemoderivative. Med Oncol. 2006;23:91–104. doi: 10.1385/MO:23:1:91. [PubMed] [Cross Ref]
- Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. oncol Rep. 2008;20:1305–1311. [PubMed]
- Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, Carroll MW. Vaccination of Colorectal Cancer Patients with Modified Vaccinia Ankara Delivering the Tumor Antigen 5T4 (TroVax) Induces Immune Responses which Correlate with Disease Control: A Phase I/II Trial. Clin Cancer Res. 2006;12:3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [PubMed] [Cross Ref]
- Speetjens FM, Kuppen PJ, Welters MJ, Essahsah F, Voet van den Brink AM, Lantrua MG, Valentijn AR, Oostendorp J, Fathers LM, Nijman HW, Drijfhout JW, van de Velde CJ, van der Melief CJ. Induction of p53-Specific Immunity by a p53 Synthetic Long Peptide Vaccine in Patients Treated for Metastatic Colorectal Cancer. Clin Cancer Res. 2009;15:1086–1091. doi: 10.1158/1078-0432.CCR-08-2227. [PubMed] [Cross Ref]
- Tamir A, Basagila E, Kagahzian A, Jiao L, Jensen S, Nicholls J, Tate P, Stamp G, Farzaneh F, Harrison P, Stauss H, George AJ, Habib N, Lechler RI, Lombardi G. Induction of tumor-specific T-cell responses by vaccination with tumor lysate-loaded dendritic cells in colorectal cancer patients with carcinoembryonic-antigen positive tumors. Cancer Immunol Immunother. 2007;56:2003–2016. doi: 10.1007/s00262-007-0299-y. [PubMed] [Cross Ref]
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I Clinical Trial of Autologous Ascites-derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol Ther. 2008;164:782–790. doi: 10.1038/mt.2008.1. [PubMed] [Cross Ref]
- Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, Schilling M, Bercovici N, Loudovaris M, Guillermo R, Lee SM, Bender J, Mills B, Fong L. Vaccination of Metastatic Colorectal Cancer Patients With Matured Dendritic Cells Loaded With Multiple Major Histocompatibility Complex Class I Peptides. J Immunother. 2007;30:762–772. doi: 10.1097/CJI.0b013e318133451c. [PubMed] [Cross Ref]
- Okaji Y, Tsuno NH, Tanaka M, Yoneyama S, Matsuhashi M, Kitayama J, Saito S, Nagura Y, Tsuchiya T, Yamada J, Tanaka J, Yoshikawa N, Nishikawa T, Shuno Y, Todo T, Saito N, Takahashi K. Pilot study of anti-angiogenic vaccine using fixed whole endothelium in patients with progressive malignancy after failure of conventional therapy. Eur J Cancer. 2008;44:383–390. doi: 10.1016/j.ejca.2007.10.018. [PubMed] [Cross Ref]
- Seledtsov VI, Niza NA, Felde MA, Shishkov AA, Samarin DM, Seledtsova GV, Seledtsov DV. Xenovaccinotherapy for colorectal cancer. Biomed Pharmacother. 2007;61:125–130. doi: 10.1016/j.biopha.2006.09.016. [PubMed] [Cross Ref]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, Oh SY, Han SY, Yoon JH, Hong SH, Moon A, Speth K, Park C, Ahn YJ, Daneshmand M, Rhee BG, Pinedo HM, Bell JC, Kirn DH. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [PubMed] [Cross Ref]
- Wittig B, Märten A, Dorbic T, Weineck S, Min H, Niemitz S, Trojaneck B, Flieger D, Kruopis S, Albers A, Löffel J, Neubauer A, Albers P, Müller S, Sauerbruch T, Bieber T, Huhn D, Schmidt-Wolf IG. Therapeutic Vaccination against Metastatic Carcinoma by Expression-Modulated and Immunomodified Autologous Tumor Cells: A First Clinical Phase I/II Trial. Hum Gene Ther. 2001;12:267–278. doi: 10.1089/10430340150218404. [PubMed] [Cross Ref]
- Menon AG, Kuppen PJ, van der Burg SH, Offringa R, Bonnet MC, Harinck BI, Tollenaar RA, Redeker A, Putter H, Moingeon P, Morreau H, Melief CJ, van de Velde CJ. Safety of intravenous administration of a canarypox virus encoding the human wild-type p53 gene in colorectal cancer patients. Cancer Gene Ther. 2003;10:509–517. doi: 10.1038/sj.cgt.7700600. [PubMed] [Cross Ref]
- Hamid O, Varterasian ML, Wadler S, Hecht JR, Benson A, Galanis E, Uprichard M, Omer C, Bycott P, Hackman RC, Shields AF. Phase II Trial of Intravenous CI-1042 in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2003;21:1498–1504. doi: 10.1200/JCO.2003.09.114. [PubMed] [Cross Ref]
- Waterston AM, Gumbrell L, Bratt T, Waller S, Gustav-Aspland J, L'hermenier C, Bellenger K, Campbell M, Powles T, Highley M, Bower M, Mouritsen S, Feldmann M, Coombes RC. Phase I study of TNFalpha AutoVaccIne in patients with metastatic cancer. Cancer Immunol Immunother. 2005;54:848–857. doi: 10.1007/s00262-005-0661-x. [PubMed] [Cross Ref]
- Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, Niedzwiecki D, Panicali D, Schlom J, Lyerly HK. Phase I Study of Immunization with Dendritic Cells Modified with Fowlpox Encoding Carcinoembryonic Antigen and Costimulatory Molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [PubMed] [Cross Ref]
- Babatz J, Röllig C, Löbel B, Folprecht G, Haack M, Günther H, Köhne CH, Ehninger G, Schmitz M, Bornhäuser M. Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer Immunol Immunother. 2006;55:268–276. doi: 10.1007/s00262-005-0021-x. [PubMed] [Cross Ref]
- Liu KJ, Wang CC, Chen LT, Cheng AL, Lin DT, Wu YC, Yu WL, Hung YM, Yang HY, Juang SH, Whang-Peng J. Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A 2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res. 2004;10:2645–2651. doi: 10.1158/1078-0432.CCR-03-0430. [PubMed] [Cross Ref]
- Zbar AP, Thomas H, Wilkinson RW, Wadhwa M, Syrigos KN, Ross EL, Dilger P, Allen-Mersh TG, Kmiot WA, Epenetos AA, Snary D, Bodmer WF. Immune responses in advanced colorectal cancer following repeated intradermal vaccination with the anti-CEA murine monoclonal antibody, PR1A3: results of a phase I study. Int J Colorectal Dis. 2005;20:403–414. doi: 10.1007/s00384-004-0726-x. [PubMed] [Cross Ref]
- Neidhart J, Allen KO, Barlow DL, Carpenter M, Shaw DR, Triozzi PL, Conry RM. Immunization of colorectal cancer patients with recombinant baculovirus-derived KSA (Ep-CAM) formulated with monophosphoryl lipid A in liposomal emulsion, with and without granulocyte-macrophage colony-stimulating factor. Vaccine. 2004;22:773–780. doi: 10.1016/j.vaccine.2003.08.021. [PubMed] [Cross Ref]
- Conry RM, Khazaeli MB, Saleh MN, Allen KO, Barlow DL, Moore SE, Craig D, Arani RB, Schlom J, LoBuglio AF. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clin Cancer Res. 1999;5:2330–2337. [PubMed]
- Morse MA, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, Schlom J, Ryback ME, Lyerly HK. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res. 1999;5:1331–1338. [PubMed]
- Samonigg H, Wilders-Truschnig M, Kuss I, Plot R, Stöger H, Schmid M, Bauernhofer T, Tiran A, Pieber T, Havelec L, Loibner H. A double-blind randomized-phase II trial comparing immunization with antiidiotype goat antibody vaccine SCV 106 versus unspecific goat antibodies in patients with metastatic colorectal cancer. J Immunother. 1999;22:481–488. doi: 10.1097/00002371-199911000-00002. [PubMed] [Cross Ref]
- Sobol RE, Shawler DL, Carson C, Van Beveren C, Mercola D, Fakhrai H, Garrett MA, Barone R, Goldfarb P, Bartholomew RM, Brostoff S, Carlo DJ, Royston I, Gold DP. Interleukin 2 gene therapy of colorectal carcinoma with autologous irradiated tumor cells and genetically engineered fibroblasts: a Phase I study. Clin Cancer Res. 1999;5:2359–2365. [PubMed]
- Woodlock TJ, Sahasrabudhe DM, Marquis DM, Greene D, Pandya KJ, McCune CS. Active specific immunotherapy for metastatic colorectal carcinoma: phase I study of an allogeneic cell vaccine plus low-dose interleukin-1 alpha. J Immuno ther. 1999;22:251–259. doi: 10.1097/00002371-199905000-00008. [PubMed] [Cross Ref]
- Hanna MG Jr, Hoover HC Jr, Pinedo HM, Finer M. Active Specific Immunotherapy with Autologous Tumor Cell Vaccines for Stage II Colon Cancer: Logistics, Efficacy, Safety and Immunological Pharmacodynamics. Hum Vaccin. 2006;2:185–191. [PubMed]
- Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ, Tollenaar RA. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:223–228. doi: 10.1056/NEJM199807233390403. [PubMed] [Cross Ref]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. [PubMed]
- von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–1191. [PubMed]
- Beck K, Blansfield J, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [PMC free article] [PubMed] [Cross Ref]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [PubMed] [Cross Ref]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2006;56:641–648. doi: 10.1007/s00262-006-0225-8. [PubMed] [Cross Ref]
- Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [PMC free article] [PubMed] [Cross Ref]
- O'Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, Gause B, Pittaluga S, Neelapu S, Brown M, Fleisher TA, Gulley JL, Schlom J, Nussenblatt R, Albert P, Davis TA, Lowy I, Petrus M, Waldmann TA, Janik JE. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. [PubMed]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [PMC free article] [PubMed] [Cross Ref]
- Idenoue S, Hirohashi Y, Torigoe T, Sato Y, Tamura Y, Hariu H, Yamamoto M, Kurotaki T, Tsuruma T, Asanuma H, Kanaseki T, Ikeda H, Kashiwagi K, Okazaki M, Sasaki K, Sato T, Ohmura T, Hata F, Yamaguchi K, Hirata K, Sato N. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res. 2005;11:1474–8142. doi: 10.1158/1078-0432.CCR-03-0817. [PubMed] [Cross Ref]
- Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [PubMed] [Cross Ref]
Gerelateerde artikelen
- crispr-cas9-bewerkte T-cellen gericht op CISH maakt patienten met gevorderde darmkanker en GIST alsnog gevoelig voor immuuntherapie met anti-PD medicijnen
- Immuuntherapie met nivolumab plus ipilimumab vooraf aan operatie bij darmkankerpatienten met hoge waarden van microsatellite-instability high/mismatch repair deficient (MSI-H/dMMR) blijkt zeer effectief.
- Immuuntherapie met Pembrolizumab geeft veel betere resultaten op ziektevrije overleving (48 vs 18 procent op 2 jaars meting) dan chemotherapie voor uitgezaaide darmkanker met MSI-H/dMMR - of afwijkende reparatie genen
- KRAS gemuteerde tumoren: Kankervaccin ELI-002 2P stimuleerde hoge T-celreacties bij patiënten met voor immuuntherapie ongevoelige KRAS-gemuteerde tumoren en verbeterde de ziekteprogressieve tijd bij patienten met alvleesklierkanker en darmkanker copy 1 co
- Immuuntherapie vooraf aan operatie en chemotherapie blijkt succesvol bij kankerpatiënten met maagkanker en met tumoren op de overgang van slokdarm naar de maag copy 2
- Dostarlimab, een specifieke vorm van een anti-PD medicijn is 100 procent effectief bij alle 12 patienten met operabele rectumkanker met dMMR = Mismatch-reparatie-deficiëntie en was geen operatie meer nodig.
- Kankerpatienten met solide tumoren met MSI-H = hoge microsatelliet instabiliteit en mismatch reparatie (dMMR) reageren uitstekend op immuuntherapie met pembrolizumab vooraf aan operatie met 65 tot 80 procent complete remissies copy 1
- Immuuntherapie met nivolumab plus ipilimuab voorafgaand aan operatie darmkanker geeft uitstekende resultaten met duurzame klinische complete remissies
- Temozolomide - temodal gevolgd door immunotherapie met combinatie van lage dosis ipilimumab plus nivolumab geeft hoopgevende resultaten bij patiënten met microsatellietstabiel en MGMT-gedempte uitgezaaide darmkanker
- CYAD-101, een vorm van CAR-T cel immuuntherapie geeft hoopvolle resultaten bij uitgezaaide darmkanker zonder dat graft-versus-host-ziekte ontstaat.
- Dendritische cellen en Newcastle Disease Virus bij kankerpatiënten met spijsverteringskanker geeft significant betere resultaten in overlevingstijd aldus gerandomiseerde studie bij 335 patiënten. copy 2
- Autologe genetisch gemodificeerde T-cellen gericht tegen het humaan papillomavirus (HPV) 16 E6 geeft bij patienten met vergevorderde zwaar voorbehandelde uitgezaaide HPV gerelateerde kanker uitstekende resultaten copy 1
- Man met uitgezaaide darmkanker stadium 4 (KRAS pos.) geneest alsnog met combinatie van immuuntherapie met Rigvir virus (dendritische celtherapie) plus FOLFOX en bevacizumab en is nu na 8 jaar kankervrij.
- Nivolumab (Opvido) + ipilimumab (Yervoy) geeft uitstekende resultaten bij nog niet behandelde uitgezaaide darmkanker (met MSI-H of dMMR mutaties) en bereikte 53 procent een gedeeltelijke remissie en 7 procent een complete remissie.
- Immuuntherapie met een gemoduleerd virus plus avelumab, een anti-PD medicijn, wordt onderzocht in een fase I/II studie bij darmkankerpatienten
- Man met uitgezaaide inoperabele darmkanker komt met ATID - Autologous tumor immunizing devascularization, een vorm van immuuntherapie in complete remissie en is al 16 jaar kankervrij, zonder chemo of andere behandelingen
- Immuuntherapie met autovaccinatie van dendritische cellen moet weer eerstelijns behandeling worden voor operabele darmkanker stadium II en III
- Immuuntherapie met dendritische celtherapie geeft uitstekende resultaten op overall overleving en ziektevrije tijd bij darmkanker met weinig of geen zichtbare tumoren.
- Xilonix - MAPp1 zorgt voor stabiele ziekte (bij 53 procent) bij zwaar voorbehandelde darmkankerpatienten stadium 4 met een mediane overall overleving van 4.2 maanden vs 11.5 maanden in vergelijking met placebo copy 1
- Dendritische cellen en Newcastle Disease Virus bij kankerpatiënten met spijsverteringskanker geeft significant betere resultaten in overlevingstijd aldus gerandomiseerde studie bij 335 patiënten. copy 1
- Immuuntherapie: Oncophage(R) (HSPPC-96), gemaakt van eigen tumorcellen zorgt voor opmerkelijke resultaten bij o.a. darmkanker.
- Immuuntherapie met het gemoduleerde virus Ankara–5T4 (TroVax) plus lage dosis cyclophosphamide zorgt voor verdubbeling van mediane overall overleving 11,2 vs 20 maanden bij vergevorderde darmkanker
- Immuuntherapie geeft uitstekende resultaten bij darmkanker met minimale ziekte - weinig tumoren - en zelfs bij darmkanker stadium IV werkt het ook al is het dan minder effectief
- Immuuntherapie met een moleculair middel - codenaam MGN1703 - geeft hoog significant betere ziektevrije tijd bij patiënten met uitgezaaide darmkanker die eerder chemotherapie kregen.
- Autovaccinatie bij darmkanker. Oncovax geeft significant beter resultaat op overleving, overlevingstijd en tijd tot recidief bij darmkankerpatiënten met stadum II. Intracel is failliet verklaard. Oncovax wordt niet meer geleverd
- Archief: immuuntherapie met CEA anti-body plus bestraling geeft hoopvolle resultaten in Phase-II trial met 30 darmkankerpatiënten.
- Immuuntherapie waaronder dendritische celtherapie bij vormen van darmkanker, een overzicht



 1,4
1,4
Plaats een reactie ...
Reageer op "Immuuntherapie geeft uitstekende resultaten bij darmkanker met minimale ziekte - weinig tumoren - en zelfs bij darmkanker stadium IV werkt het ook al is het dan minder effectief"