CONTACT
Zen Logsdon
626-409-9367
zlogsdon@coh.org
The Phase 1 clinical trial tests the safety of providing City of Hope-developed AOH1996 to people with reoccurring solid tumors.
LOS ANGELES — City of Hope, one of the largest cancer research and treatment organizations in the United States, today announced that the first patient to receive its novel, promising cancer medicine AOH1996 is doing well. The Phase 1 clinical trial testing the safety of a potentially cancer-stopping therapeutic developed by City of Hope in people with reoccurring solid tumors is expected to continue for the next two years. The investigational pill has been effective in preclinical research treating cells derived from breast, prostate, brain, ovarian, cervical, skin and lung cancers.
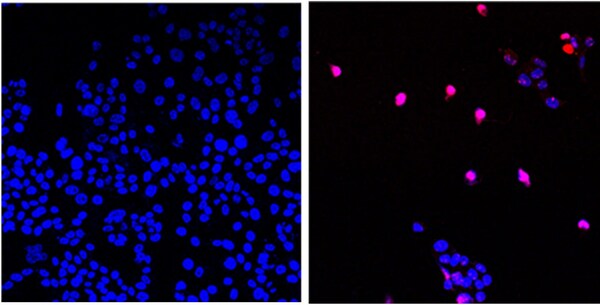
Linda Malkas, Ph.D., professor in City of Hope’s Department of Molecular Diagnostics & Experimental Therapeutics, has been working on the research and subsequent discovery and development of AOH1996 for 20 years. AOH1996 is named after Anna Olivia Healey, a young girl born in 1996 who unfortunately was not able to beat cancer. AOH1996 is exclusively licensed by City of Hope to RLL, LLC, a biotechnology company that Malkas co-founded.
Malkas believed that proliferating cell nuclear antigen (PCNA), which plays an essential role in the replication and repair of cells, would be a less toxic cancer therapy that targets mutated cancer cells while leaving normal cells alone. The treatment has been shown in preclinical research to target PCNA and inhibit the growth and spread of a broad range of human cancer cells. The research protocol notes that AOH1996 is not toxic to healthy cells and that treatment with this medicine both pauses cell DNA synthesis and inhibits DNA repair, leading to a type of cell death known as apoptosis in the cancer cells.
“Imagine cancer as the water filling up a bathtub. Left unchecked, the tumors or water will eventually overflow and damage other parts of your home. The treatment my team at City of Hope created is akin to a watchful homeowner who shuts the water off — stopping the spread of tumors to other parts of the metaphorical house — and then drains the tub, eliminating the cancer,” said Malkas, co-investigator in the trial and the M.T. & B.A. Ahmadinia Professor in Molecular Oncology.
Vincent Chung, M.D., added, “By targeting PCNA, we are inhibiting the complex machinery to stop cellular growth and proliferation. This is a new way of trying to kill cancer cells or at least to slow it down.” He is a research professor in City of Hope’s Department of Medical Oncology & Therapeutics Research and principal investigator in the clinical trial.
The Phase 1 clinical trial is open at City of Hope Los Angeles. Its objective is to determine the maximum tolerated dose of the investigational pill, AOH1996, and to evaluate the medicine for preliminary efficacy. Eligible patients include adults with solid tumors who have not found standard treatments effective. Participating patients will be asked to take the medication in pill form twice a day.
“Since many patients’ cancers become resistant to our standard therapies, we need new therapeutics with new mechanisms of action — for example, non-cross resistant. AOH1996 is just that kind of new therapy,” said Daniel Von Hoff, M.D., of the Molecular Medicine Division at the Translational Genomics Research Institute, part of City of Hope, and an advisor on the study. “Congratulations to Dr. Malkas and the City of Hope team on this new invention.”
Malkas said other targeted therapies, like checkpoint inhibitors, that inhibit the growth and spread of cancer have helped innumerable cancer patients, adding that perhaps one day AOH1996 will be a U.S. Food and Drug Administration-approved inhibitor that could be used in combination with existing therapies to both enhance cancer-killing effects as well as decrease side effects related to lifesaving cancer treatments.
With the infrastructure and support of City of Hope, Malkas was able to commercialize her basic research, moving her promising laboratory discovery into a clinical trial for people who need the therapies of tomorrow today. City of Hope provided structure, experts and even GMP (good manufacturing practice) facilities to manufacture her medicine.
City of Hope is devoted to supporting research and development and has infrastructure that is tailor-made to accelerate innovations from the lab to patients. This system includes everything ranging from funding support to access to counsel from internal and external drug development specialists. City of Hope's R&D apparatus leverages the combination of its fundamental strength in basic science and its patient-care focus as a comprehensive cancer center.
Individuals interested in this clinical trial should review the eligibility requirements at clinicaltrials.gov. If they believe they are eligible, they can call 626-218-1133 or visit City of Hope’s clinical trials webpage.




Plaats een reactie ...
Reageer op "Kankerpil - AOH1996, van professor Linda Malkas die alle solide tumoren zou vernietigen is aan eerste patient gegeven in fase I studie"