A novel endoscopic electroporation procedure eliminated the need for insulin when combined with semaglutide in patients with type 2 diabetes, according to the results of the first-in-human EMINENT study being presented during Digestive Disease Week® (DDW) 2023.
“This is one of the first times that endoscopy has been used to improve glycemic control in patients with type 2 diabetes,” said Jacques Bergman, MD, PhD, principal investigator on the study and professor of gastrointestinal endoscopy at Amsterdam University Medical Center. “Using a single outpatient procedure combined with semaglutide, we have shown that it may be possible to either reduce or eliminate the need for insulin.”
The procedure, dubbed re-cellularization via electroporation therapy (ReCETTM), uses electroporation to ablate the superficial layer of the mucosa in the small intestine. The hope is that rejuvenation of the duodenal lining combined with the use of a GLP-1 receptor agonist will reduce insulin resistance in patients with type 2 diabetes, thus addressing the root cause of disease, researchers said.
Unlike other ablation methods, ReCET does not use extreme heat or cold to damage the intestinal tissue. “ReCET uses a pulsed electric field, which can be precisely controlled and has a limited depth of penetration,” said Celine Busch, MD, the study’s lead researcher and PhD candidate at the medical center. “It doesn’t directly physically damage the mucosa. Instead, it disrupts the cellular membrane of the mucosa and superficial submucosa. These cells subsequently die of natural cell death within 24 hours of the procedure. There is no increase in temperature during the procedure, which significantly reduces the chance of complications.”
EMINENT was a single-arm pilot study of 14 patients with type 2 diabetes using long-acting basal insulin to control their blood glucose levels. All patients underwent the hour-long ReCET procedure and started on semaglutide two weeks later.
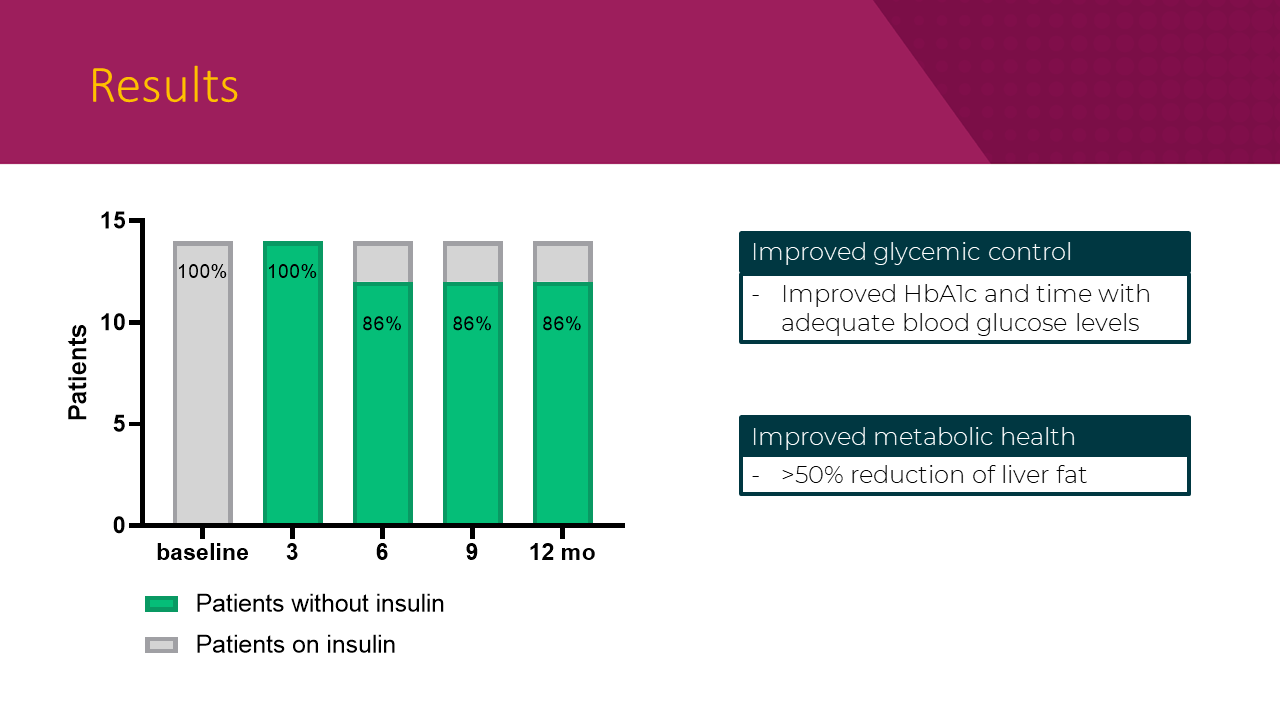
Overall, ReCET was shown to be feasible and safe, researchers said. The procedure was successful in 100% of patients, and no patients experienced serious adverse events associated with endoscopy. The majority of patients (93%) were able to tolerate the maximum dose of semaglutide.
Key efficacy results after twelve months of follow-up include:
- 86% of patients (12 out of14) were able to stay off insulin while maintaining glycemic control.
- Fasting plasma glucose levels improved from 158 mg/dl to 119 mg/dl.
- HbA1c improved from 7.2% to 6.6% (p=0.010).
- > 50% reduction in liver fat percentage.
- Other metabolic parameters, such as cholesterol, low-density lipoprotein (LDL), and triglyceride levels showed a trend toward improvement, but the changes were not statistically significant.
“One of the biggest advantages is that this treatment is compliance-free,” said Dr. Busch. “Patients with type 2 diabetes are generally on six to eight different drugs, and many are not taking these drugs correctly.”
Due to the single-arm nature of the study, it is difficult to tease out the impact of ReCET and that of semaglutide on glycemic control, but Dr. Busch noted that there is reason to believe that the two approaches may be working together.
“In most studies in which patients on long-acting insulin receive a GLP-1 receptor agonist, less than 20% can stop insulin completely,” she said. “It’s difficult to explain our results based on just the activity of semaglutide. We know that GLP-1 receptor agonists boost the release of endogenous insulin. This acts synergistically with the insulin-sensitizing effect of ReCET, as reflected in a 86% success rate at 6, 9 and 12 months follow-up.”
Dr. Bergman’s group is planning a randomized controlled trial to confirm their results.
The study was fully funded by Endogenex, a Minnesota-based company that owns the technology used for the endoscopic procedure. Dr. Bergman serves on the advisory board of Endogenex.
Dr. Busch’s oral presentation, “Re-cellularization via electroporation therapy (ReCET) combined with GLP-1RA to replace insulin therapy in patients with Type 2 diabetes: Twelve-month results of the EMINENT study,” abstract 1272, on Tuesday, May 9, at 4:22 p.m. CDT, is part of the session “ASGE Progress In Foregut Endoscopy.”



Plaats een reactie ...
Reageer op "ReCET - electroporation therapy is een nieuwe endoscopische behandeling en verbetert glykemische controle en stopt behoefte aan medisch toepassen van insuline bij patiënten met diabetes type 2"