Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
12 jun i 2016: zie ook dit artikel:
12 juni 2016: Op Asco 2016 is een meta analyse gepresenteerd die bij elkaar heeft gebracht in de meting van kwaliteit van leven en pijnervaringen hoe patienten met uitgezaaide hormoonresistente prostaatkanker hun behandelingen hebben ervaren met chemo, abiraterone, enzalutamide, Radium 223 en andere nieuwe medicijnen / behandelingen waaronder ook immuuntherapie met Provenge - sipuleucel-T. De meta analyse is gedaan bij studies met gelijk of meer deelnemers van 50 patienten waarbij de doelen, een primair doel of secondair doel de kwaliteit van leven en / of vermindering van pijn waren.
In de conclusie wordt nadrukkelijk vermeld dat een vergelijking van de verschillende behandelingen op kwaliteit van leven en pijnvermindering niet mogelijk is en elke individuele studie zo zijn eigen resultaten laat zien. In het studierapprot verwijzen bepaalde resutlaten altijd naar de nummering van de onderzochte studies in de referentielijst.
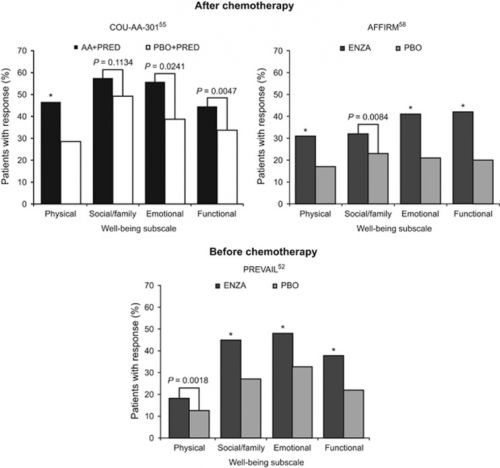
Hier twee van de vele tabellen en grafieken. Figuur 2 geeft overzicht van middelen zoals door de onderzoekers genoemd: niet-toxische aanpak met abiraterone en enzalutamide.
De grafiek daaronder figuur 2 geeft de totaalscore van alle gemeten medicijnen en behandelingen. In het volledige studierapport staan veel meer grafieken en tabellen.
Figure 2.
Proportion of patients with metastatic castration-resistant prostate cancer reporting a clinically meaningful improvement on the FACT-P subscale well-being scores after receipt of noncytotoxic therapies. AA, abiraterone acetate; ENZA, enzalutamide; FACT-P, Functional Assessment of Cancer Therapy-Prostate; PBO, placebo; PRED, prednisone. *P<0.0001 vs comparator.
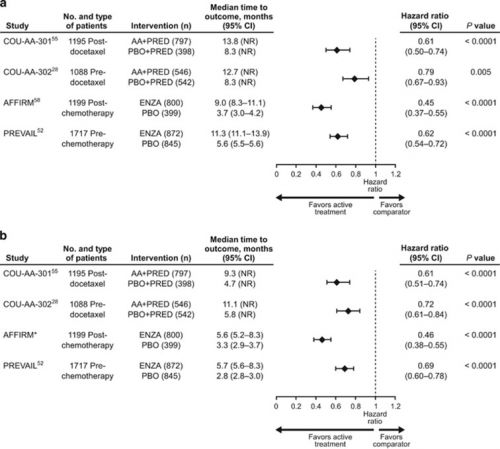
De studie geeft zoveel meer duidelijke cijfers en tabellen dat het niet zinnig is die hier nog een keer te vermelden. Het volledige studierapport: Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science is met de tabellen en grafieken gratis in te zien. Onderaan dit artikel het abstract en bijbehorende referentielijst.
Recently published randomized clinical trials of new agents for mCRPC have captured elements of the patient experience while on treatment. Further research is required to standardize methods for measuring, quantifying and reporting on HRQoL and pain in patients with mCRPC in the clinical practice setting.
Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science.
Abstract
BACKGROUND:
Contemporary therapies for metastatic castration-resistant prostate cancer (mCRPC) have shown survival improvements, which do not account for patient experience and health-related quality of life (HRQoL).
METHODS:
This literature review included a search of MEDLINE for randomized clinical trials enrolling ⩾50 patients with mCRPC and reporting on patient-reported outcomes (PROs) since 2010.
RESULTS:
Nineteen of 25 publications describing seven treatment regimens (10 clinical trials and nine associated secondary analyses) met the inclusion criteria and were critically appraised. The most commonly used measures were the Functional Assessment of Cancer Therapy-Prostate (n=5 trials) and Brief Pain Inventory Short Form (n=4 trials) questionnaires. The published data indicated that HRQoL and pain status augmented the clinical efficacy data by providing a better understanding of treatment impact in mCRPC. Abiraterone acetate and prednisone, enzalutamide, radium-223 dichloride and sipuleucel-T offered varying levels of HRQoL benefit and/or pain mitigation versus their respective comparators, whereas three treatments (mitoxantrone, estramustine phosphate and docetaxel, and cabazitaxel) had no meaningful impact on HRQoL or pain. The main limitation of the data were that the PROs utilized were not developed for use in mCRPC patients and hence may not have comprehensively captured symptoms important to this population.
CONCLUSIONS:
Recently published randomized clinical trials of new agents for mCRPC have captured elements of the patient experience while on treatment. Further research is required to standardize methods for measuring, quantifying and reporting on HRQoL and pain in patients with mCRPC in the clinical practice setting.
- PMID:
- 26832363
- [PubMed - in process]
- PMCID:
- PMC4868871
-
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [PubMed]
- Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol 2001; 165: 1146–1151. [PubMed]
- Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1-T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer 2004; 100: 1283–1292. [PubMed]
- Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA et al. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys 2003; 57: 915–928. [PubMed]
- Kupelian PA, Thakkar VV, Khuntia D, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: long-term outcomes. Int J Radiat Oncol Biol Phys 2005; 63: 1463–1468. [PubMed]
- Beyer DC. Brachytherapy for recurrent prostate cancer after radiation therapy. Semin Radiat Oncol 2003; 13: 158–165. [PubMed]
- Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003; 169: 517–523. [PubMed]
- Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007; 51: 1175–1184. [PubMed]
- Rosenbaum E, Partin A, Eisenberger MA. Biochemical relapse after primary treatment for prostate cancer: studies on natural history and therapeutic considerations. J Natl Compr Canc Netw 2004; 2: 249–256. [PubMed]
- Kirby M, Hirst C, Crawford ED. Characterising the castration resistant prostate cancer population: a systematic review. Int J Clin Pract 2011; 65: 1180–1192. [PubMed]
- Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989; 321: 419–424. [PubMed]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al.; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. [PubMed]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520. [PubMed]
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L et al.; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002; 94: 1458–1468. [PubMed]
- Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol 2009; 27: 5431–5438. [PubMed]
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al.; Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [PMC free article] [PubMed]
- U.S. Department of Health and Human Services. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available at http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf (accessed on 16 June 2015). [PMC free article] [PubMed]
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, ; CONSORT PRO Group. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013; 309: 814–822. [PubMed]
- Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14: 1756–1764. [PubMed]
- Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993; 25: 805–813. [PubMed]
- Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol 1998; 16: 1574–1581. [PubMed]
- Berry DL, Moinpour CM, Jiang CS, Ankerst DP, Petrylak DP, Vinson LV et al. Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol 2006; 24: 2828–2835. [PubMed]
- Colloca G, Venturino A, Checcaglini F. Patient-reported outcomes after cytotoxic chemotherapy in metastatic castration-resistant prostate cancer: a systematic review. Cancer Treat Rev 2010; 36: 501–506. [PubMed]
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS et al.; PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [PMC free article] [PubMed]
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ et al.; COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [PubMed]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF et al.; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422. [PubMed]
- Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15: 738–746. [PubMed]
- Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014; 66: 815–825. [PMC free article] [PubMed]
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al.; COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [PMC free article] [PubMed]
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al.; AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [PubMed]
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN et al.; COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. [PubMed]
- Hoskin P, Sartor O, O'Sullivan JM, Johannessen DC, Helle SI, Logue J et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014; 15: 1397–1406. [PubMed]
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I et al.; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. [PubMed]
- Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 2012; 30: 4249–4255. [PubMed]
- Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol 2015; 26: 179–185. [PMC free article] [PubMed]
- Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 2012; 30: 1534–1540. [PMC free article] [PubMed]
- Meulenbeld HJ, van Werkhoven ED, Coenen JL, Creemers GJ, Loosveld OJ, de Jong PC et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic Castration Resistant Prostate Cancer (CRPC), the Netherlands Prostate Study (NePro). Eur J Cancer 2012; 48: 2993–3000. [PubMed]
- Dawson N, Payne H, Battersby C, Taboada M, James N. Health-related quality of life in pain-free or mildly symptomatic patients with metastatic hormone-resistant prostate cancer following treatment with the specific endothelin A receptor antagonist zibotentan (ZD4054). J Cancer Res Clin Oncol 2011; 137: 99–113. [PubMed]
- Quinn DI, Tangen CM, Hussain M, Lara PN Jr, Goldkorn A, Moinpour CM et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol 2013; 14: 893–900. [PMC free article] [PubMed]
- Fizazi K, Higano CS, Nelson JB, Gleave M, Miller K, Morris T et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2013; 31: 1740–1747. [PubMed]
- Basch E, Autio KA, Smith MR, Bennett AV, Weitzman AL, Scheffold C et al. Effects of cabozantinib on pain and narcotic use in patients with castration-resistant prostate cancer: results from a phase 2 nonrandomized expansion cohort. Eur Urol 2015; 67: 310–318. [PubMed]
- Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy-Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009; 12: 124–129. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [PubMed]
- Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997; 50: 920–928. [PubMed]
- Cleeland C. The Brief Pain Inventory: User Guide. Available at http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf (accessed on 16 June 2015).
- Small EJ, Higano CS, Kantoff PW, Whitmore JB, Frohlich MW, Petrylak DP. Time to disease-related pain and first opioid use in patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Prostate Cancer Prostatic Dis 2014; 17: 259–264. [PMC free article] [PubMed]
- Nilsson S, Strang P, Aksnes AK, Franzèn L, Olivier P, Pecking A et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 2012; 48: 678–686. [PubMed]
- Bahl A, Oudard S, Tombal B, Ozgüroglu M, Hansen S, Kocak I et al.; TROPIC Investigators. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol 2013; 24: 2402–2408. [PMC free article] [PubMed]
- Caffo O, Sava T, Comploj E, Fariello A, Zustovich F, Segati R et al. Impact of docetaxel-based chemotherapy on quality of life of patients with castration-resistant prostate cancer: results from a prospective phase II randomized trial. BJU Int 2011; 108: 1825–1832. [PubMed]
- Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD et al.; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [PubMed]
- Joly F, Delva R, Mourey L, Sevin E, Bompas E, Vedrine L et al. Clinical benefits of non-taxane chemotherapies in unselected symptomatic metastatic castration-resistant prostate cancer patients after docetaxel: the Getug P02 study. BJU Int 2015; 115: 65–73. [PubMed]
- Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol 2015; 16: 509–521. [PubMed]
- Sternberg CN, Molina A, North S, Mainwaring P, Fizazi K, Hao Y et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol 2013; 24: 1017–1025. [PubMed]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al.; COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [PMC free article] [PubMed]
- Harland S, Staffurth J, Molina A, Hao Y, Gagnon DD, Sternberg CN et al.; COU-AA-301 Investigators. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer 2013; 49: 3648–3657. [PubMed]
- Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol 2012; 13: 1210–1217. [PubMed]
- Basch E, Autio K, Ryan CJ, Mulders P, Shore N, Kheoh T et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol 2013; 14: 1193–1199. [PubMed]
- Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, Cella D et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 2014; 15: 1147–1156. [PubMed]
- Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol 2013; 24: 31–38. [PubMed]
- Eton DT, Shevrin DH, Beaumont J, Victorson D, Cella D. Constructing a conceptual framework of patient-reported outcomes for metastatic hormone-refractory prostate cancer. Value Health 2010; 13: 613–623. [PubMed]
- Sullivan PW, Mulani PM, Fishman M, Sleep D. Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone-refractory prostate cancer. Qual Life Res 2007; 16: 571–575. [PubMed]
- Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 2002; 288: 3027–3034. [PubMed]
- Cella D, Ivanescu C, Phung D, Mansbach H, Holmstrom S, Naidoo S Impact on quality of life of enzalutamide or abiraterone acetate+prednisone in the treatment of metastatic castration-resistant prostate cancer that has progressed on or after doxetaxel—a comparative effectiveness study. Presented at International Society for Pharmacoeconomics and Outcomes Research 20th Annual International Meeting, 16–20 May 2015, Philadelphia, PA, USA 2015.
- World Health Organization. Palliative care: symptom management and end-of-life care. Available at http://www.who.int/3by5/publications/documents/en/genericpalliativecare082004.pdf (accessed on 16 June 2015).
- Ringash J, O'Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer 2007; 110: 196–202. [PubMed]
- Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 2005; 28: 172–191. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [PubMed]
- Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol 1997; 50: 441–450. [PubMed]
- Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S. Test/retest study of the European Organization for research and treatment of cancer core quality-of-life questionnaire. J Clin Oncol 1995; 13: 1249–1254. [PubMed]
- Osoba D, Tannock IF, Ernst S, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol 1999; 17: 1654–1663. [PubMed]
- Borghede G, Sullivan M. Measurement of quality of life in localized prostatic cancer patients treated with radiotherapy. Development of a prostate cancer-specific module supplementing the EORTC QLQ-C30. Qual Life Res 1996; 5: 212–222. [PubMed]
- Maringwa J, Quinten C, King M, Ringash J, Osoba D, Coens C et al.; EORTC PROBE Project and Brain Cancer Group. Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol 2011; 22: 2107–2112. [PubMed]
- The EuroQol group. EuroQol-a new facility for the measurement of health related quality of life. Health Policy 1990; 16: 199–208. [PubMed]
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–343. [PubMed]
- Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res 1993; 2: 169–180. [PubMed]
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [PMC free article] [PubMed]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999; 85: 1186–1196. [PubMed]
- Farrar JT, Rk Portenoy, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain 2000; 88: 287–294. [PubMed]
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975; 1: 277–299. [PubMed]
- Melzack R. The McGill Pain Questionnaire: from description to measurement. Anesthesiology 2005; 103: 199–202. [PubMed]
- Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995; 61: 277–284. [PubMed]
- Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock AI, ; TAX-327 investigators. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain and quality of life response and survival in the TAX-327 Study. Clin Cancer Res 2008; 14: 2763–2767. [PubMed]
- Petrioli R, Fiaschi AI, Pozzessere D, Messinese S, Sabatino M, Marsili S et al. Weekly epirubicin in patients with hormone-resistant prostate cancer. Br J Cancer 2002; 87: 720–725. [PMC free article] [PubMed]
Gerelateerde artikelen
- ESMO 2024: aanbevolen abstracten gerelateerd aan prostaatkanker gepresenteerd in Barcelona van 13 tot 17 september 2024
- ASCO GU 2025 Genitourinary Cancers Symposium: overzicht van gepresenteerde abstracten gerelateerd aan prostaatkanker, peniskanker, nierkanker en zaadbalkanker
- Parpremmers bij prostaatkanker, een overzicht
- Reviewstudie met de effecten van nieuwe levensverlengende geneesmiddelen voor patiënten met uitgezaaide castratieresistente prostaatkanker (mCRPC) wereldwijd
- Waaraan moet een ziekenhuis voldoen om prostaatkanker te mogen behandelen? Zie hier de SONCOS normen
- Richtlijnen voor het systemisch behandelen van uitgezaaide prostaatkanker vastgesteld na meta analyse van 26 gerandomiseerde studies
- Nieuwe richtlijnen van The American Urological Association (AUA) voor het behandelen van patiënten met hormoon resistente vormen van gevorderde uitgezaaide prostaatkanker.
- Prostaatkankerpatienten met een risico op hart- en vaatziektes en/of met gemiddeld risico op progressie van prostaatkanker kunnen beter naast bestraling geen hormoontherapie gebruiken.
- Follow-up of Prostatectomy versus Observation for Early Prostate Cancer
- Abiraterone Acetate door FDA officieel goedgekeurd om te gebruiken als medicijn bij gevorderde prostaatkanker
- Androgeenreceptorremmers: apalutamide, enzalutamide en daralutamide geven betere overall overleving dan een placebo bij mannen met niet uitgezaaide maar wel hormoonresistente prostaatkanker. . Zelfs bij mannen ouder dan 80 jaar
- Apalutamide plus hormoontherapie vermindert het risico op tweede recidief of overlijden (min 33 procent) ongeacht hormoontherapie of chemotherapie bij patiënten met uitgezaaide hormoonresistente prostaatkanker
- Atrasentran remt bij 20 procent van de prostaatkankerpatiënten de kankergroei bij uitgezaaide prostaatkanker aldus fase 3 studie.
- Bestraling - radiotherapie bij prostaatkanker: een overzicht van belangrijke studies en recente ontwikkelingen
- AR-V7 receptor voorspelt welke behandeling zinvol is voor hormoonresistente gevorderde prostaatkanker: chemo of abiraterone en enzalutamide copy 1
- Chemo: Taxotere tegenover Mitoxantrone in combinatie met prednison - verlengt leven van prostaatkankerpatiënten met gemiddeld drie maanden, maar chemo bij prostaatkanker is geen effectieve behandeling
- Chirurgie + radiotherapie geeft betere 10-jaars overleving in vergelijking met radiotherapie plus hormoontherapie voor prostaatkanker met aangetaste lymfklieren, maar geeft wel meer bijwerkingen.
- Cribriform: Prostaatkankerpatienten met cribriform positief hebben grotere kans op uitzaaiingen op afstand in vergelijking met prostaatkankerpatienten die cribriform negatief testen
- Cryosurgery bij recidief van prostaatkanker geeft betere kosten - baten analyse dan hormoontherapie en zelfde resultaten op overleving met betere kwaliteit van leven
- Darolutamide (Nubeqa) blijkt samen met hormoontherapie betere resultaten te geven dan chemo met docetaxel bij uitgezaaide castratiegevoelige prostaatkanker bewijst fase III studie Aranote
- De lust en last van de Prostaat? Een podcast die mensen met vragen over de prostaat en alles daaromheen iets verder kan helpen. Van Dr. Melianthe Nicolai en therapeut Jeroen de Haas
- Diagnose: overzicht van artikelen en informatie van diagnose technieken bij verdenking van prostaatkanker
- DNA mutaties zoals ATM, BRCA1, BRCA2 zijn al te traceren bij diagnose van uitgezaaide prostaatkanker en hebben voorspellende waarde op overall overleving
- durvalumab plus olaparib na resistentie voor abiraterone of enzalutamide geeft alsnog mediaan minimaal een jaar progressieve ziekte nagenoeg zonder bijwerkingen bij in botten uitgezaaide prostaatkanker
- Enzalutamide - Xtandi bij prostaatkanker, een overzicht van publicaties
- HIFU - Ultra Sound als pijnbestrijding voor in botten uitgezaaide prostaatkanker in UMC Utrecht neemt nog steeds deelnemers aan. copy 1
- Hormoontherapie artikelen in een overzicht bij elkaar gezet
- Immuuntherapeutische aanpak, waaronder dendritische celtherapie bij prostaatkanker: een overzicht van belangrijke studies en recente ontwikkelingen.
- Kwaliteit van leven en pijnvermindering bij uitgezaaide hormoonresistente prostaatkanker vanuit patientenervaringen geanalyseerd- een meta analyse
- Nanoknife - Irreversibele Elektroporatie Therapie (IRE) wordt ook bij prostaatkanker toegepast in St. Antonius Ziekenhuis
- Observatie: Een wait and see beleid bij prostaatkanker is vaak beter - in 30 tot 50 procent van de gevallen - dan een directe behandeling inclusief operatie en is nu bevestigd door een grote Europese studie o.a. uitgevoerd door het Erasmus Medisch Centrum
- Operatietechnieken bij prostaatkanker: een overzicht van recente ontwikkelingen en relevante studies
- Patient met prostaatkanker stadium IV reageert uitzonderlijk goed op trastuzumab deruxtecan (T-DXd) waar elke andere aanpak faalde en is nog steeds in leven en in relatief goede gezondheid
- PDT - Photodynamische Therapie, met ook enkele studies bij prostaatkankerpatiënten succesvol uitgevoerd
- Radium-223 samen met abiraterone of enzalutamine geeft langere overall overleving dan alleen Radium-223 of alleen andere behandelingen bij uitgezaaide vergevorderde prostaatkanker
- Radicale ingreep - operatie of intensieve bestraling van prostaatkanker met hoog risico geeft 50 procent minder kans op overlijden in vergelijking met alleen hormoontherapie
- Relugolix geeft snellere onderdrukking van de testosteronspiegel in vergelijking met leuproleline (lucrin) voor patienten met gevorderde prostaatkanker met veel lager risico op ernstige cardiovasculaire bijwerkingen
- Statine gebruik alleen of in combinatie met metformine geeft prostaatkankerpatienten met hoog risico factoren 32 procent minder kans om te overlijden aan alle oorzaken en 54 procent minder kans te overlijden specifiek aan prostaatkanker.
- Velcade - Bortezomib - onder de codenaam MLN2704 toont nu ook uitstekende resultaten bij prostaatkankerpatiënten met recidief van gevorderde prostaatkanker.
- Zometa - Zoledronic Acid geeft betere resultaten dan APD - Pamidronate bij botproblemen vanuit prostaatkanker
- Reguliere oncologie: overzicht van recente ontwikkelingen en belangrijke studies binnen de reguliere oncologie voor prostaatkanker



Plaats een reactie ...
Reageer op "Kwaliteit van leven en pijnvermindering bij uitgezaaide hormoonresistente prostaatkanker vanuit patientenervaringen geanalyseerd- een meta analyse"