Raadpleeg ook onze lijst van niet-toxische ondersteuning bij prostaatkanker. :
Update 30 augustus 2017:
Op ASCO 2017 werd deze studie: Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017
gepubliceerd waarin 60 specialisten op het gebied van prostaatkanker hun mening geven over 10 discussiegebieden bij de behandeling van prostaatkanker in verschillende stadia. Opvallend dat bij veel discussiepunten er behoorlijk verschillend werd gedacht over voorgestelde behandelingen en er voor veel punten geen consensus was. Ik ga de studie niet vertalen, daarvoor is het teveel en u kunt de vertaling gebruiken van goolge translation rechtsboven dit artikel. Nogmaals zie dit studierapport dat volledig gratis is in te zien:
26 augustus 2017: Bron: Journal of Clinical Oncology and National Cancer Institute
Vorig jaar publiceerde het NCI een studie: Short Androgen Suppression and Radiation Dose Escalation for Intermediate- and High-Risk Localized Prostate Cancer: Results of EORTC Trial 22991 waaruit bleek dat prostaatkankerpatiënten gebaat zijn met een korte periode van hormoontherapie naast bestraling - radiotherapie. 6 maanden aanvullend hormoontherapie zou voldoende zijn.
Nu komt het NCI met aanvullende gegevens en richtlijnen voor de uroloog en oncoloog wanneer wel en niet hormoontherapie zinvol is. Patiënten (60 plus) met een Gleasonscore van 7 of minder bij de diagnose en/of prostaatkankerpatiënten (alle leeftijden) met een groter risico op hart- en vaatziektes zouden beter geen hormoontherapie kunnen krijgen naast bestraling. Het risico om te overlijden aan hartfalen zou groter zijn dan overlijden aan prostaatkanker.
Dit blijkt uit een gedetailleerde analyse van de patienten die wel of niet profiteerden van hormoontherapie.
Hier een grafiek uit deze studie: Weighing Risk of Cardiovascular Mortality Against Potential Benefit of Hormonal Therapy in Intermediate-Risk Prostate Cancer
Tekst loopt verder onder grafieken. Eerste grafiek geeft de risicofactoren aan.
Table 1. Distribution of individual cardiac risk factors as defined in Framingham Heart Study (33)*
| Risk factors | Definitions |
|---|---|
| Blood pressure, % | |
| Optimal | Systolic < 120 mm Hg; diastolic < 80 mm Hg |
| Borderline | Systolic 120–139 mm Hg or diastolic 80–89 mm Hg |
| High | Systolic ≥140 mm Hg, diastolic ≥90 mm Hg, or treatment for hypertension |
| Serum LDL cholesterol level, % | |
| Optimal | <2.59 mmol/L (<100 mg/dL) |
| Borderline | 2.59–4.12 mmol/L (100–159 mg/dL) |
| High | 4.12 mmol/L (>159 mg/dL) |
| Serum HDL cholesterol level, % | |
| Optimal | >1.53 mmol/L (>59 mg/dL) |
| Borderline | 1.04–1.53 mmol/L (40–59 mg/dL) |
| Low | <1.04 mmol/L (<40 mg/dL) |
| Glucose tolerance, % | |
| Optimal | Fasting glucose level < 6.11 mmol/L (<110 mg/dL) or 2 h glucose level < 7.77 mmol/L (<140 mg/dL) |
| Borderline | Fasting glucose level 6.11–6.94 mmol/L (110–125 mg/dL) or 2-h glucose level 7.77–11.04 mmol/L (140–199 mg/dL) |
| High | Known diabetes or fasting glucose level > 6.94 mmol/L (>125 mg/dL) or 2 h glucose level >11.04 mmol/L (>199 mg/dL) |
| Smoking, % | |
| Optimal | Never |
| Borderline | Former |
| High | Current |
*HDL = high-density lipoprotein; LDL = low-density lipoprotein.
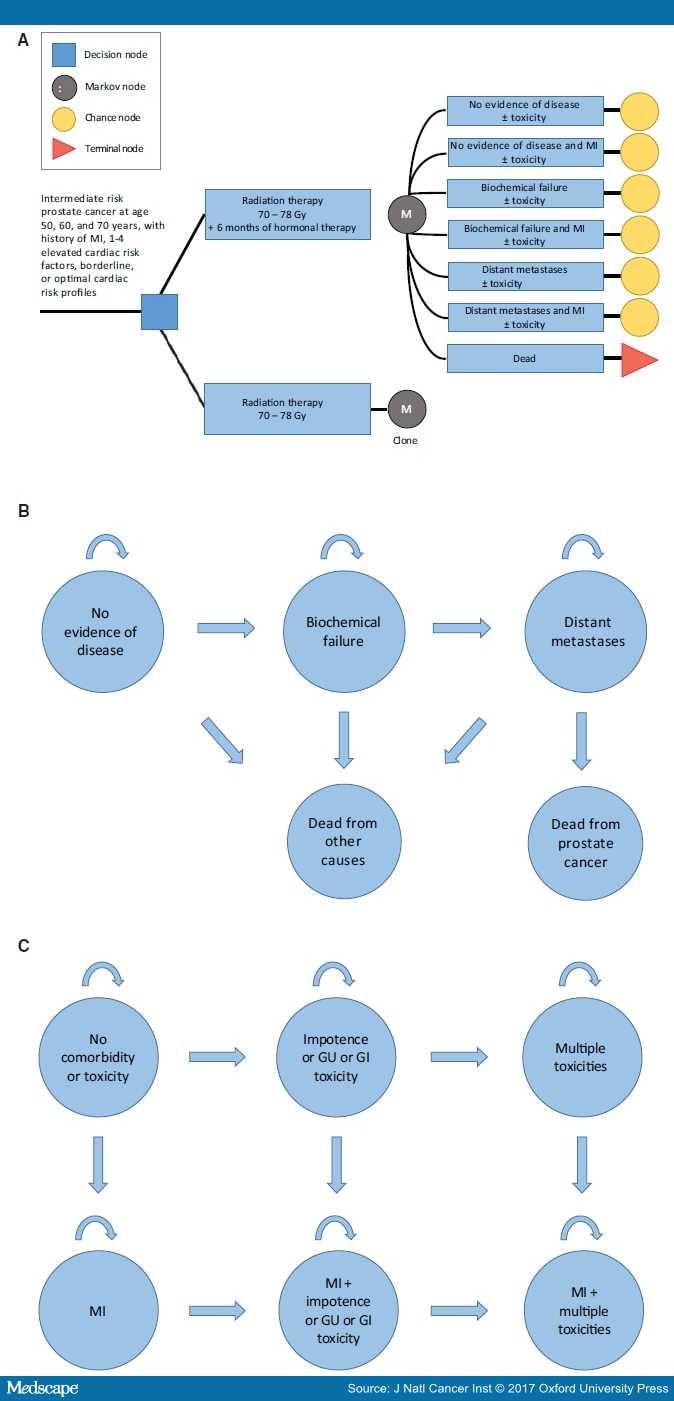
Figure 1.
Schematic overviews of the model. A) Abbreviated decision tree and Markov model used to compare two strategies for treating intermediate-risk prostate cancer as done in the EORTC22991 trial. B) Influence diagram shows the network of five disease-related health states and comorbidity and toxicity health states (C) linked by transitional variables. GI = gastrointestinal; GU = genitourinary; MI = myocardial infarction.
The primary end point is quality-adjusted life expectancy. Men transition between different health states, including no evidence of disease, biochemical failure, and distant metastases (Figure 1B).
Het NCI heeft samen met Medscape een model gemaakt dat richtlijnen aangeeft voor urologen en oncologen wanneer het aan een prostaatkankerpatient wel en wanneer het beter is geen hormoontherapie te geven.
Als u de hele tekst wilt lezen dan klik op deze link: Weighing Risk of Cardiovascular Mortality Against Potential Benefit of Hormonal Therapy in Intermediate-Risk Prostate Cancer
Hier de conclusie tekst met referentielijst:
In conclusion, we found that most men with intermediate-risk prostatecancer are expected to benefit from HT if they survive beyond seven years. However, patients with multiple cardiac risk factors may have a decrement in survival with HT. In the absence of randomized data, the current study suggests that younger patients with no cardiac risk factors should receive RT with HT. However, patients at very low risk of biochemical failure or who have a history of MI should be treated with RT alone regardless of cardiac risk.
Discussion
The EORTC 22991 trial demonstrates a clear PC control benefit by adding HT to dose-escalated RT with regard to bDFS compared with RT alone, with 7.2 years' median follow-up. While OS data are not yet mature, it is possible that with longer follow-up a survival benefit will be observed with HT, given that improvements in bDFS can result in an OS benefit. It remains to be determined, however, which subgroups of patients with intermediate-risk PC benefit most from HT. This is of particular interest given the recently proposed subdivisions of intermediate-risk disease into favorable and unfavorable groups based on Gleason score pattern, PSA, and percent positive cores.[48–54] Furthermore, the presence of clinically significant comorbidity, and in particular cardiovascular disease, may offset the benefit of HT either because of the marginal benefit in a patient with limited overall life expectancy and/or a true negative interaction between cardiovascular disease and HT. Additionally, HT is associated with statistically significant adverse side effects, including fatigue and sexual dysfunction. This was further demonstrated in the EORTC 22991 trial as sexual function was more severely affected in patients who received RT + HT compared with RT alone (27.0% vs 19.4%, P = 0.010).
Given the complicated nature of patient identification and treatment selection, decision analyses can help clinicians strategize by comparing risks and benefits associated with different treatment options. Therefore, we created a decision analysis to replicate outcomes of the EORTC 22992 study and to generate expected outcomes with additional follow-up after stratifying men by age and cardiovascular risk. In our model, a benefit to HT was not observed until just beyond the median follow-up of the EORTC 22992 (7.2 years). This suggests that benefits of adding six months of HT to RT are expected to manifest with longer follow-up, with the exception of men with a history of MI, where no benefit was observed with lifetime follow-up. Our model also showed that the healthiest men with the longest life expectancy benefited the most from HT. For instance, the magnitude of benefit was much larger in men age 50 years with optimal risk compared with men age 70 years with four cardiac risk factors (2.6 vs 0.4 QALYs). These results can be explained by the greater competing risks of death and shorter life expectancy in older men with cardiac risk factors.
When exploring ranges of values on sensitivity analyses, the results were consistent over a wide variety of assumptions. We found that HT is not likely to improve QALYs in men at low risk for biochemical failure (≤8.7% at five years). In a retrospective analysis that stratified patients treated with dose-escalated RT with or without HT into favorable intermediate-risk, marginal, and unfavorable intermediate-risk groups, the five-year bDFS for patients treated with RT alone was 94% in the favorable-risk group compared with 74% in unfavorable-risk group. Therefore, our analysis suggests that men with intermediate-risk PC with a low risk of biochemical failure—similar to favorable intermediate-risk disease—achieve optimal QALYs with RT alone, regardless of the number of cardiac risk factors. Using our model to extrapolate beyond what is reported in the EORTC 22991 trial, the men most likely to benefit from HT include those with unfavorable intermediate-risk PC and up to four cardiac risk factors. Conversely, men with favorable intermediate-risk disease or those with history of MI are unlikely to benefit. Sensitivity analyses also revealed that the up-front, transient side effects from HT are negated by avoiding lifetime HT at the time of recurrence in men who received RT with HT. This should be considered during the decision-making process in candidates for HT.
Men with a history of MI have a shorter life expectancy relative to men without previous MI. Therefore, in our model, harms associated with HT—including unwanted side effects and the potential increased risks of cardiac mortality—outweigh improvements in PCSM in men with clinically significant cardiovascular disease. HT was observed to negatively impact men with a history of MI (−0.3 to −0.4 QALYs) because the reductions in QALYs from HT were more apparent in men with the shortest life expectancy. Similarly, when 0.5 utility was assumed for the no evidence of disease health state in average-risk men in our model, the benefits of HT were reduced (1.1 vs 0.3 QALYs).
While several population-based studies have showed positive associations between HT and both the incidence of cardiovascular disease and worsened death from cardiovascular disease,[18,19,21,23,25] postrandomization analyses have produced conflicting data. While some analyses suggest increased risk of MI and lack of benefit of HT,[27,58] others do not report an increased risk of MI and cardiovascular mortality in men with PC who received HT.[59–61] The discrepancy among these studies is perhaps explained by the fact that the prospective randomized trials did not evaluate cardiovascular outcomes in men with a documented history of MI.[1–7,62] For example, in the meta-analysis by Nguyen et al., trials included in the analysis did not stratify by preexisting cardiovascular comorbidity.
Our study is consistent with population-based analyses, which show that reductions in PC mortality from HT are offset by net harm in men with baseline cardiovascular disease.[18–27] Whether HT is necessary for favorable intermediate-risk PC will be addressed by RTOG 0815. In the absence of mature data from this trial, the current study and other retrospective series demonstrate that dose-escalated RT may be sufficient for patients with intermediate-risk PC and cardiovascular risk factors.[48–53]
This study has several limitations. First, the underlying risk of MI development and mortality was derived from the Framingham Heart Study, which was undertaken prior to introduction of sophisticated cardiovascular imaging and percutaneous coronary intervention. The present study suggests that we should incorporate a more sophisticated cardiac risk stratification scheme that includes noninvasive anatomic and physiologic assessment of coronary artery disease.
Second, we did not model four months of HT, an option for men with intermediate-risk PC based on the RTOG 9910 randomized trial. However, this trial did not incorporate dose escalation, and it may be underpowered in men with unfavorable intermediate-risk PC who might benefit from more than four months of HT. Specifically, the proportion of men in RTOG 9910 with favorable intermediate-risk PC, a subgroup of intermediate-risk PC where HT may not be necessary to reduce the risk of PCSM, is unknown.
Third, there is uncertainty inherent to quality of life estimates. In our study, there were no thresholds found for health state utilities. The utility estimates were based on a widely cited source of 162 men age 60 years or older (52% of whom had been diagnosed with prostate cancer) who were highly motivated volunteers. Finally, a Markov model is not a substitute for a prospective randomized controlled trial. A randomized trial comparing HT + RT to RT alone in patients stratified by both favorable and unfavorable intermediate-PC risk and cardiac risk is the optimal method of assessing the impact of HT on cardiovascular outcomes.
In conclusion, we found that most men with intermediate-risk PC are expected to benefit from HT if they survive beyond seven years. However, patients with multiple cardiac risk factors may have a decrement in survival with HT. In the absence of randomized data, the current study suggests that younger patients with no cardiac risk factors should receive RT with HT. However, patients at very low risk of biochemical failure or who have a history of MI should be treated with RT alone regardless of cardiac risk.
References
-
Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527.
-
Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073.
-
Crook J, Ludgate C, Malone S, et al. Final report of multicenter Canadian phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(2):327–333.
-
Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12(5):451–459.
-
Laverdière J, Gomez JL, Cusan L, et al. Beneficial effect of combination hormonal therapy administered prior and following external beam radiation therapy in localized prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37(2):247–252.
-
Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997;15(3):1013–1021.
-
Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet. 2009;373(9660):301–308.
-
Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(3):320–327.
-
D'Amico AV, Manola J, Loffredo M, et al. Six-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA. 2004;292(7):821–827.
-
Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118.
-
Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(4):980–988.
-
Beckendorf V, Guerif S, Le Prise E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063.
-
Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–473.
-
Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74.
-
Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28(7):1106–1111.
-
Martinez AA. A phase III prospective randomized trial of dose-escalated radiotherapy with or without short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0815.AccessedMay 14, 2016.
-
Bolla M, Maingon P, Carrie C, et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC Trial 22991. J Clin Oncol. 2016;34(15):1748–1756.
-
Keating NL, O'Malley aJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46.
-
Nanda A, Chen M-H, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866–873.
-
Punnen S, Cooperberg MR, Sadetsky N, et al. Androgen deprivation therapy and cardiovascular risk. J Clin Oncol. 2011;29:3510–3516.
-
Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500.
-
Smith MR, Klotz L, van der Meulen E, et al. Gonadotropin-releasing hormone blockers and cardiovascular disease risk: Analysis of prospective clinical trials of degarelix. J Urol. 2011;186:1835–1842.
-
Tsai HK, D'Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524.
-
Nguyen PL, Chen MH, Goldhaber SZ, et al. Coronary revascularization and mortality in men with congestive heart failure or prior myocardial infarction who receive androgen deprivation. Cancer. 2011;117:406–413.
-
O'Farrell S, Garmo H, Holmberg L, et al. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243–1251.
-
Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67(6):1009–1016.
-
D'Amico AV, Chen MH, Renshaw A, et al. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314(12):1291–1293.
-
Conteduca V, Di Lorenzo G, Tartarone A, et al. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: an unresolved controversy. Crit Rev Oncol Hematol. 2013;86(1):42–51.
-
Lester JF, Mason MD. Cardiovascular effects of hormone therapy for prostate cancer. Drug Healthc Patient Saf. 2015;7:129–138.
-
Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: Critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98(6):3589–3593.
-
Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–743.
-
Lester-Coll NH, Goldhaber SZ, Sher DJ, et al. Death from high-risk prostate cancer versus cardiovascular mortality with hormonal therapy: A decision analysis. Cancer. 2013;119(10):1808–1815.
-
Vasan RS, Sullivan LM, Wilson PWF, et al. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann Int Med. 2005;142(6):393–402.
-
D'Amico AV, Chen M-H, Renshaw Aa, et al. Interval to testosterone recovery after hormonal therapy for prostate cancer and risk of death. Int J Radiat Oncol Biol Phys. 2009;75(1):10–15.
-
Kaku H, Saika T, Tsushima T, et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate. 2006;66(4):439–444.
-
Stout NK, Knudsen AB, Kong CY, et al. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics. 2009;27(7):533–545.
-
Pound CR, Partin aW, Eisenberger Ma, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597.
-
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223.
-
Armstrong PW, Fu Y, Chang WC, et al. Acute coronary syndromes in the GUSTO-IIb trial: Prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation. 1998;98(18):1860–1868.
-
Howlader N, Noone AM, Krapcho M, et al SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. Accessed May 15, 2016.
-
Keenan NL, Shaw KM. Coronary heart disease and stroke deaths - United States, 2006. MMWR. Surveillance summaries: Morbidity and mortality weekly report. Surveillance summaries/CDC. MMWR Morb Mortal Wkly Rep. 2011;60(suppl):62–66.
-
Xu J, Kochanek KD, Murphy SL, et al. Deaths: Final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–136.
-
National Cancer Institute. Common terminology criteria for adverse events v 4.0. May 29, 2009. NIH publication # 09-7473.
-
Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: A decision analysis. JAMA. 2010;304(21):2373–2380.
-
Stewart ST, Lenert L, Bhatnagar V, et al. Utilities for prostate cancer health states in men aged 60 and older. Med Care. 2005;43(4):347–355.
-
Lazar LD, Pletcher MJ, Coxson PG, et al. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–153.
-
Abramowitz MC, Li T, Buyyounouski MK, et al. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer. 2008;112(1):55–60.
-
Castle KO, Hoffman KE, Levy LB, et al. Is androgen deprivation therapy necessary in all intermediate-risk prostate cancer patients treated in the dose escalation era? Int J Radiat Oncol Biol Phys. 2013;85(3):693–699.
-
Keane FK, Chen MH, Zhang D, et al. The likelihood of death from prostate cancer in men with favorable or unfavorable intermediate-risk disease. Cancer. 2014;120(12):1787–1793.
-
Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64(6):895–902.
-
Zumsteg ZS, Zelefsky MJ. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: The standard of care? Lancet Oncol. 2012;13(6):e259–e269.
-
Dinh KT, Muralidhar V, Mahal BA, et al. Occult high-risk disease in clinically low-risk prostate cancer with >/=50% positive biopsy cores: Should national guidelines stop calling them low risk? Urology. 2016;87:125–132.
-
Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58(6):843–848.
-
Raldow AC, Zhang D, Chen MH, et al. Short-course androgen deprivation therapy and the risk of death from high-risk prostate cancer in men undergoing external beam radiation therapy and brachytherapy. Brachytherapy. 2015;14(6):781–787.
-
Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: Results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19(17):3750–3757.
-
Detsky AS, Naglie G, Krahn MD, et al. Primer on medical decision analysis: Part 1–Getting started. Med Decis Making. 1997;17(2):123–125.
-
Bucholz EM, Normand SL, Wang Y, et al. Life expectancy and years of potential life lost after acute myocardial infarction by sex and race: A cohort-based study of Medicare beneficiaries. J Am Coll Cardiol. 2015;66(6):645–655.
-
D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25(17):2420–2425.
-
Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: Analysis of RTOG 92-02. Eur Urol. 2008;54(4):816–823.
-
Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27(1):92–99.
-
Nguyen PL, Je Y, Schutz Y, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: A meta-analysis of randomized trials. JAMA. 2011;306(21):2359–2366.
-
D'Amico AV, Chen MH, Renshaw Aa, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA. 2008;299(3):289–295.
-
Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol. 2015;33(4):332–339.
-
D'Amico AV. Personalizing the duration of androgen-deprivation therapy use in the management of intermediate-risk prostate cancer. J Clin Oncol. 2015;33(4):301–303.
J Natl Cancer Inst. 2016;109(6) © 2016 Oxford University Press
Gerelateerde artikelen
- ESMO 2024: aanbevolen abstracten gerelateerd aan prostaatkanker gepresenteerd in Barcelona van 13 tot 17 september 2024
- ASCO GU 2025 Genitourinary Cancers Symposium: overzicht van gepresenteerde abstracten gerelateerd aan prostaatkanker, peniskanker, nierkanker en zaadbalkanker
- Parpremmers bij prostaatkanker, een overzicht
- Reviewstudie met de effecten van nieuwe levensverlengende geneesmiddelen voor patiënten met uitgezaaide castratieresistente prostaatkanker (mCRPC) wereldwijd
- Waaraan moet een ziekenhuis voldoen om prostaatkanker te mogen behandelen? Zie hier de SONCOS normen
- Richtlijnen voor het systemisch behandelen van uitgezaaide prostaatkanker vastgesteld na meta analyse van 26 gerandomiseerde studies
- Nieuwe richtlijnen van The American Urological Association (AUA) voor het behandelen van patiënten met hormoon resistente vormen van gevorderde uitgezaaide prostaatkanker.
- Prostaatkankerpatienten met een risico op hart- en vaatziektes en/of met gemiddeld risico op progressie van prostaatkanker kunnen beter naast bestraling geen hormoontherapie gebruiken.
- Follow-up of Prostatectomy versus Observation for Early Prostate Cancer
- Abiraterone Acetate door FDA officieel goedgekeurd om te gebruiken als medicijn bij gevorderde prostaatkanker
- Androgeenreceptorremmers: apalutamide, enzalutamide en daralutamide geven betere overall overleving dan een placebo bij mannen met niet uitgezaaide maar wel hormoonresistente prostaatkanker. . Zelfs bij mannen ouder dan 80 jaar
- Apalutamide plus hormoontherapie vermindert het risico op tweede recidief of overlijden (min 33 procent) ongeacht hormoontherapie of chemotherapie bij patiënten met uitgezaaide hormoonresistente prostaatkanker
- Atrasentran remt bij 20 procent van de prostaatkankerpatiënten de kankergroei bij uitgezaaide prostaatkanker aldus fase 3 studie.
- Bestraling - radiotherapie bij prostaatkanker: een overzicht van belangrijke studies en recente ontwikkelingen
- AR-V7 receptor voorspelt welke behandeling zinvol is voor hormoonresistente gevorderde prostaatkanker: chemo of abiraterone en enzalutamide copy 1
- Chemo: Taxotere tegenover Mitoxantrone in combinatie met prednison - verlengt leven van prostaatkankerpatiënten met gemiddeld drie maanden, maar chemo bij prostaatkanker is geen effectieve behandeling
- Chirurgie + radiotherapie geeft betere 10-jaars overleving in vergelijking met radiotherapie plus hormoontherapie voor prostaatkanker met aangetaste lymfklieren, maar geeft wel meer bijwerkingen.
- Cribriform: Prostaatkankerpatienten met cribriform positief hebben grotere kans op uitzaaiingen op afstand in vergelijking met prostaatkankerpatienten die cribriform negatief testen
- Cryosurgery bij recidief van prostaatkanker geeft betere kosten - baten analyse dan hormoontherapie en zelfde resultaten op overleving met betere kwaliteit van leven
- Darolutamide (Nubeqa) blijkt samen met hormoontherapie betere resultaten te geven dan chemo met docetaxel bij uitgezaaide castratiegevoelige prostaatkanker bewijst fase III studie Aranote
- De lust en last van de Prostaat? Een podcast die mensen met vragen over de prostaat en alles daaromheen iets verder kan helpen. Van Dr. Melianthe Nicolai en therapeut Jeroen de Haas
- Diagnose: overzicht van artikelen en informatie van diagnose technieken bij verdenking van prostaatkanker
- DNA mutaties zoals ATM, BRCA1, BRCA2 zijn al te traceren bij diagnose van uitgezaaide prostaatkanker en hebben voorspellende waarde op overall overleving
- durvalumab plus olaparib na resistentie voor abiraterone of enzalutamide geeft alsnog mediaan minimaal een jaar progressieve ziekte nagenoeg zonder bijwerkingen bij in botten uitgezaaide prostaatkanker
- Enzalutamide - Xtandi bij prostaatkanker, een overzicht van publicaties
- HIFU - Ultra Sound als pijnbestrijding voor in botten uitgezaaide prostaatkanker in UMC Utrecht neemt nog steeds deelnemers aan. copy 1
- Hormoontherapie artikelen in een overzicht bij elkaar gezet
- Immuuntherapeutische aanpak, waaronder dendritische celtherapie bij prostaatkanker: een overzicht van belangrijke studies en recente ontwikkelingen.
- Kwaliteit van leven en pijnvermindering bij uitgezaaide hormoonresistente prostaatkanker vanuit patientenervaringen geanalyseerd- een meta analyse
- Nanoknife - Irreversibele Elektroporatie Therapie (IRE) wordt ook bij prostaatkanker toegepast in St. Antonius Ziekenhuis
- Observatie: Een wait and see beleid bij prostaatkanker is vaak beter - in 30 tot 50 procent van de gevallen - dan een directe behandeling inclusief operatie en is nu bevestigd door een grote Europese studie o.a. uitgevoerd door het Erasmus Medisch Centrum
- Operatietechnieken bij prostaatkanker: een overzicht van recente ontwikkelingen en relevante studies
- Patient met prostaatkanker stadium IV reageert uitzonderlijk goed op trastuzumab deruxtecan (T-DXd) waar elke andere aanpak faalde en is nog steeds in leven en in relatief goede gezondheid
- PDT - Photodynamische Therapie, met ook enkele studies bij prostaatkankerpatiënten succesvol uitgevoerd
- Radium-223 samen met abiraterone of enzalutamine geeft langere overall overleving dan alleen Radium-223 of alleen andere behandelingen bij uitgezaaide vergevorderde prostaatkanker
- Radicale ingreep - operatie of intensieve bestraling van prostaatkanker met hoog risico geeft 50 procent minder kans op overlijden in vergelijking met alleen hormoontherapie
- Relugolix geeft snellere onderdrukking van de testosteronspiegel in vergelijking met leuproleline (lucrin) voor patienten met gevorderde prostaatkanker met veel lager risico op ernstige cardiovasculaire bijwerkingen
- Statine gebruik alleen of in combinatie met metformine geeft prostaatkankerpatienten met hoog risico factoren 32 procent minder kans om te overlijden aan alle oorzaken en 54 procent minder kans te overlijden specifiek aan prostaatkanker.
- Velcade - Bortezomib - onder de codenaam MLN2704 toont nu ook uitstekende resultaten bij prostaatkankerpatiënten met recidief van gevorderde prostaatkanker.
- Zometa - Zoledronic Acid geeft betere resultaten dan APD - Pamidronate bij botproblemen vanuit prostaatkanker
- Reguliere oncologie: overzicht van recente ontwikkelingen en belangrijke studies binnen de reguliere oncologie voor prostaatkanker



Plaats een reactie ...
Reageer op "Prostaatkankerpatienten met een risico op hart- en vaatziektes en/of met gemiddeld risico op progressie van prostaatkanker kunnen beter naast bestraling geen hormoontherapie gebruiken."