Helpt u ons aan 500 donateurs?
Lees ook:
20 april 2018: lees ook dit artikel:
16 januari 2018: Lees ook dit artikel
1 september 2017: Bron: The Lancet
Pembrolizumab (Keytruda), zowel 2 wekelijks als drie wekelijks blijkt ook bij de definitieve analyse van de Kyenote-006 studie) betere resultaten te geven in vergelijking met ipilimumab op overall overleving en ziektevrije tijd bij gevorderde inoperabele melanomen stadium III en IV.
Na mediaan bijna twee jaar (22,9 maanden) follow-up bleek 55% van de melanoompatienten die pembrolizumab hadden gekregen nog in leven in vergelijking met 43% van de patiënten die ipilimumab hadden gekregen.
Uit het studierapport:
.jpg)
Zie verderop in dit artikel gedetailleerde beschrijving van de studie. Het nieuwe studierapport is gepubliceerd in The Lancet en is tegen betaling in te zien: Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006)
Findings
Between Sept 18, 2013, and March 3, 2014, 834 patients with advanced melanoma were enrolled and randomly assigned to receive intravenous pembrolizumab every 2 weeks (n=279), intravenous pembrolizumab every 3 weeks (n=277), or intravenous ipilimumab every 3 weeks (ipilimumab for four doses; n=278). One patient in the pembrolizumab 2 week group and 22 patients in the ipilimumab group withdrew consent and did not receive treatment. A total of 811 patients received at least one dose of study treatment. Median follow-up was 22·9 months; 383 patients died. Median overall survival was not reached in either pembrolizumab group and was 16·0 months with ipilimumab (hazard ratio 0·68, 95% CI 0·53–0·87 for pembrolizumab every 2 weeks vs ipilimumab; p=0·0009 and 0·68, 0·53–0·86 for pembrolizumab every 3 weeks vs ipilimumab; p=0·0008). 24-month overall survival rate was 55% in the 2-week group, 55% in the 3-week group, and 43% in the ipilimumab group.
De studie staat geregistreerd in ClinicalTrials.gov, onder nummer NCT01866319.
12 mei 2015: Bron: NEJM 2015 Apr 19. [Epub ahead of print]
Pembrolizumab (Keytruda), een anti-PD medicijn blijkt superieure resultaten te geven in vergelijking met ipilimumab (Yervoy), een vorm van immuuntherapie in de behandeling van inoperabele vergevorderde melanomen. Aldus blijkt uit tussenresultaten van de wereldwijd uitgevoerde fase III KEYNOTE-006 studie (834 patienten uit 83 ziekenhuizen in 16 verschillende landen).
Pembrolizumab verbeterde significant overall overleving , progressie-tijd, en overall response vergeleken met ipilimumab, wat nu nog de standaard behandeling is voor dit stadium van de ziekte bij vergevorderde melanomen.
Na een mediane follow-up van 13,8 maanden de 1-jaars overall overleving waren 74.1% (voor 2 wekelijkse dosis en 68.4% voor de 3-wekelijkse dosis in de pembrolizumab groupen versus 58.2% voor ipilimumab, Dit betekent statistisch significant een verbetering met 37% in overleving (P = .00052 and P = .00358, respectievelijk).

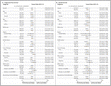
Bovendien gaf pembrolizumab minder bijwerkingen en toxicieit, zeker de ernstigere bijwerkingen die soms kunnen optreden bij ipilimumab.
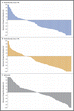
Pembrolizumab is een zogeheten checkpoint remmer, een anti-PD medicijn, zoals ook nivolumab dat is. In feite zetten deze medicijnen het immuunsysteem weer aan waar het niet functioneerde / faalde bij het ontstaan van tumorcellen. M.i. ligt de oplossing van kanker heel dichtbij.
De tussenresultaten van de KEYNOTE-006 studie zijn nu al gepubliceerd in The New England Journal of Medicine en is gepresenteerd op 19 april op ASCO 2015
Pembrolizumab wordt nieuwe standaard behandeling:
De KEYNOTE-006 studie is gestopt omdat de resultaten dermate groot waren dat het ethisch niet verantwoord is om patiënten ipilimumab te geven waar een ander medicijn veel betere resultaten geeft.
“De data uit de KEYNOTE-006 zal de standaard zorg voor inoperabele en/of gevorderde melanomen, stadium III en IV veranderen. ook voor die patiënten die al ipilimumab hebben gekregen.” aldus senior auteur van de studie, Antoni Ribas, MD, PhD, Professor of Medicine and Hematology/Oncology and Director of Tumor Immunology at the Jonsson Comprehensive Cancer Center, University of California, Los Angeles.
“Nog niet zo lang geleden discussieërden we over behandelingen die 1 op de 10 patiënten zou kunnen helpen. Met de ontwikkeling van nieuwe medicijnen die het immuunsysteem opnieuw activeren praten we over 1 op de 3 patiënten. Dat wordt de nieuwe standaard,” aldus dr. Antoni Ribas.
Ipilimumab, is een anti–cytotoxische T-lymphocyte gerelateerd eiwit 4 (CTLA-4) behandeling en was het eerste medicijn van zijn soort dat overall overleving verbeterde bij patieënten met gevorderde melanomen en het werd de standaard behandeling. Pembrolizumab is een anti–PD medicijn (anti-programmed cell death) (PD-1) antibody. Beiden zijn zogeheten immuun checkpoint remmers.
Twee anti–PD-1 remmers, pembrolizumab en nivolumab (Opdivo), zijn door de FDA de Food and Drug Administration (FDA) goedgekerud als behandeling voor melanomen als ipilimumab resistentie laat zien en als de receptoren BRAF V600 mutatie–positief zijn, na behandeling met een BRAF remmer.
Studieresultaten (heb ik nog niet vertaald maar komt uit artikel in ASCO Post:
KEYNOTE-006 included 834 patients from 83 sites in 16 different countries with unresectable stage III or IV advanced melanoma and no more than one prior systemic therapy. Patients were excluded if they received previous therapy with CTLA-4, PD-1, or PD-L1 inhibitors. Study participants were randomly assigned 1:1:1 to pembrolizumab at 10 mg/kg every 2 weeks (n = 279), pembrolizumab at 10 mg/kg every 3 weeks (n = 277), or four cycles of ipilimumab at 3 mg/kg every 3 weeks (n = 278).
Dr. Ribas explained that at the time KEYNOTE-006 was designed, the best dosing regimen of pembrolizumab was not known. The currently approved dosing regimen for pembrolizumab is 2 mg/kg every 3 weeks.
“There seems to be little difference between these dosing regimens [ie, all three pembrolizumab regimens],” he noted, “and there is no evidence that one of the two dosing regimens in KEYNOTE-006 is better.”
At a median follow-up of 7.9 months, median progression-free survival was 5.5 months for the every-2-week pembrolizumab group and 4.1 months for the every-3-week group, vs 2.8 months for ipilimumab, reflecting a 42% reduction in risk of disease progression with pembrolizumab (P < .00001).
Estimated 6-month progression-free survival rates were 47.3%, 46.4%, and 26.5%, respectively. “Progression-free survival is close to double with pembrolizumab,” Dr. Ribas noted.
At a median follow-up of 13.8 months, 1-year overall survival rates were 74.1% and 68.4% for the 2-week and 3-week pembrolizumab groups, respectively, vs 58.2% for ipilimumab, reflecting a 37% improvement in survival that was statistically significant (P = .00052 and P = .00358, respectively).
“This study exceeded our expectations, even for the control arm,” he said.
Objective response rates according to RECIST criteria for tumor shrinkage were about 33% in the pembrolizumab arms vs 11.9% in the ipilimumab arm. Complete response rates were 5%, 6.1%, and 1.4%, respectively. Responses were ongoing in 89.4% of the 2-week pembrolizumab group, 96.7% of the 3-week pembrolizumab group, and 87.9% of the ipilimumab group.
Improved Tolerability
“The toxicity profile favored pembrolizumab, with fewer side effects even though there was greater continuous exposure to the drug than with ipilimumab,” Dr. Ribas commented. Grade 3/4 toxicities were reported in 19% of the ipilimumab-treated patients and in 10% to 13% of those receiving pembrolizumab.
Treatment-related autoimmune- or immune-related adverse events with pembrolizumab were hypothyroidism (10.1% for the 2-week group and 8.7% for the 3-week group) and hyperthyroidism (6.5% and 2.5%, respectively). Colitis was reported in 8.2% of those assigned to ipilimumab.
Disclosure: Dr. Ribas has consulted for Merck, with the honoraria paid to his institution.
Het volledige studierapport: Robert C, Schachter J, Long GV, et al: Pembrolizumab versus ipilimumab in advanced melanoma is gratis in te zien op de website van de N Engl J Med. April 19, 2015 (early release online).
Het abstract staat hieronder:
The anti-PD-1 antibody pembrolizumab prolonged progression-free survival and overall survival and had less high-grade toxicity than did ipilimumab in patients with advanced melanoma.
Pembrolizumab versus Ipilimumab in Advanced Melanoma.
Abstract
Background:
The immune checkpoint inhibitor ipilimumab is the standard-of-care treatment for patients with advanced melanoma. Pembrolizumab inhibits the programmed cell death 1 (PD-1) immune checkpoint and has antitumor activity in patients with advanced melanoma.
Methods:
In this randomized, controlled, phase 3 study, we assigned 834 patients with advanced melanoma in a 1:1:1 ratio to receive pembrolizumab (at a dose of 10 mg per kilogram of body weight) every 2 weeks or every 3 weeks or four doses of ipilimumab (at 3 mg per kilogram) every 3 weeks. Primary end points were progression-free and overall survival.
Results:
The estimated 6-month progression-free-survival rates were 47.3% for pembrolizumab every 2 weeks, 46.4% for pembrolizumab every 3 weeks, and 26.5% for ipilimumab (hazard ratio for disease progression, 0.58; P<0.001 for both pembrolizumab regimens versus ipilimumab; 95% confidence intervals , 0.46 to 0.72 and 0.47 to 0.72, respectively). Estimated 12-month survival rates were 74.1%, 68.4%, and 58.2%, respectively (hazard ratio for death for pembrolizumab every 2 weeks, 0.63; 95% CI, 0.47 to 0.83; P=0.0005; hazard ratio for pembrolizumab every 3 weeks, 0.69; 95% CI, 0.52 to 0.90; P=0.0036). The response rate was improved with pembrolizumab administered every 2 weeks (33.7%) and every 3 weeks (32.9%), as compared with ipilimumab (11.9%) (P<0.001 for both comparisons). Responses were ongoing in 89.4%, 96.7%, and 87.9% of patients, respectively, after a median follow-up of 7.9 months. Efficacy was similar in the two pembrolizumab groups. Rates of treatment-related adverse events of grade 3 to 5 severity were lower in the pembrolizumab groups (13.3% and 10.1%) than in the ipilimumab group (19.9%).
Conclusions:
The anti-PD-1 antibody pembrolizumab prolonged progression-free survival and overall survival and had less high-grade toxicity than did ipilimumab in patients with advanced melanoma. (Funded by Merck Sharp & Dohme; KEYNOTE-006 ClinicalTrials.gov number, NCT01866319 .).
- PMID:
- 25891173
- [PubMed - as supplied by publisher]
Gerelateerde artikelen
- Poeptransplantatie - Fecale Microbiota Transplantatie (FMT) vergroot effectiviteit van immuuntherapie bij niet-kleincellige longkanker en melanomen copy 1
- TIL - Tumorinfiltrerende Lymfocyten therapie met Amtagvi - lifileucel geeft alsnog uitstekende resultaten bij patienten met gevorderde zwaar voorbehandelde melanomen waar eerder immuuntherapie met anti-PD medicijnen faalde
- mRNA-4157 (V940) in combinatie met pembrolizumab geeft veel langere recidiefvrije overleving en minder uitzaaiingen op afstand bij patiënten met hoogrisico stadium III/IV melanoom na operatie in vergelijking met alleen pembrolizumab
- RP1, een gemodificeerd herpes virus, geeft in combinatie met nivolumab alsnog uitstekende resultaten bij patienten met uitgezaaide melanoom waar eerder anti-PD immuuntherapie faalde
- Immuuntherapie met pembrolizumab of ipilimumab voor patienten met melanomen stadium IV en III inoperabel geeft overall overleving van 45 procent op 10 jaars meting, aldus de resultaten uit de Keynote 006 studie
- Immuuntherapie vooraf aan operatie geeft sterk verbeterde resultaten op overall overleving en minder recidieven bij patienten met een melanoom
- Uitgezaaide oogmelanoom bevat soms immuuncelkenmerken die patienten gevoelig kan maken voor TIL therapie (adoptieve tumor-infiltrerende lymfocyten - TIL)
- Mediterraan dieet stimuleert effectiviteit van immuuntherapie met anti-PD medicijnen - checkpointremmers voor patiënten met een melanoom in een gevorderd stadium copy 1
- Tumor-infiltrerende lymfocyten therapie (TIL) verbetert progressievrije overleving en mediane overall overleving bij patiënten met gevorderd melanoom (Stadium IIIc - IV) in vergelijking met standaard immunotherapie met ipilimumab.
- Vrouw met een progressief stadium IIIB-melanoom behandeld met oncolytisch ECHO-7-virus = RIGVIR virus komt in totale remissie en is nu al 10 jaar klinisch kankervrij na 1e diagnose
- Pembrolizumab gegeven na operatie voor melanoompatienten met stadium IIB en IIC verminderde het risico op overlijden en recidief met 35 procent in vergelijking met een placebo
- Relatlimab plus nivolumab geeft in vergelijking met alleen nivolumab langere ziektevrije tijd bij eerstelijns gevorderde melanoom
- Nivolumab plus ipilimumab geeft betere mediane overall overleving (72 maanden vs 37 maanden) dan alleen nivolumab of alleen ipilimumab bij patienten met inoperabele melanomen.
- Pembrolizumab geeft betere ziektevrije tijd en minder bijwerkingen in vergelijking met hoge dosis interferon (HDI) of ipilimumab bij patiënten met een geopereerd melanoom met een hoog risico op een recidief
- Chronische immuungerelateerde bijwerkingen komen veelvuldig voor bij patiënten met stadium III-IV melanoom die worden behandeld met immuuntherapie met anti-PD medicijnen
- Fecale microbiota transplantatie - FMT overwint resistentie tegen anti-PD-1-therapie bij melanoompatiënten en zorgt alsnog voor aanslaan van immuuntherapie met anti-PD medicijnen
- Immuuntherapie met nivolumab plus ipilimumab geeft veel betere overall ziektevrije overleving (plus 28 en 38 procent) in vergelijking met alleen nivolumab of placebo bij patiënten met operabele stadium IV melanoom zonder bewijs van ziekte na operatie
- Melanoompatiënten (stadium IV) die na een gerichte behandeling toch ziekteprogressie tonen, hebben baat bij daarna immuuntherapie met anti-PD-1 medicijnen en gelijke overall overleving als bij eerstelijns met anti-PD-1 medicijnen.
- Er is een sterk positief verband met het aantal immuun-gerelateerde bijwerkingen (irAE's) en recidiefvrije overleving bij melanoompatiënten met een hoog risico in stadium III die werden behandeld met pembrolizumab.
- Immuuntherapie met T-VEC = talimogene laherparepvec voor huidkanker waaronder melanomen wordt ook in Nederland toegepast in studieverband. Bv. In UMCG Groningen
- Melanoom tumoren met V600K BRAF mutatie reageren minder goed op gerichte behandelingen dan met V600E mutaties, maar V600K BRAF geeft betere resultaten op immuuntherapie met anti-PD medicijnen copy 1
- Biomarkers zoals PD-L1, CD163+ en NRAS mutaties en gegevens zoals uitzaaiingen later ontstaan bepalen kans van effectiviteit van immuuntherapie met anti PD medicijnen bij melanomen copy 1
- Immuuntherapie met pembrolizumab voorkomt veel beter recidief (met 43 procent) van operabele melanoom stadium III in vergelijking met placebo ook zonder PD-1 mutatie
- Bepaalde darmbacteriën kunnen de effectiviteit van immunotherapie met anti-PD medicijnen verhogen bij de behandeling van melanomen copy 1
- Pembrolizumab superieur in ziektevrije tijd, overall overleving en met significant minder bijwerkingen dan Ipilimumab bij inoperabele gevorderde melanomen.
- Immuuntherapie met een gepersonaliseerd vaccin geeft uitstekende resultaten bij operabele melanoompatienten, meer dan de helft bereikte een duurzame complete remissie copy 1
- Immuuntherapie met gemoduleerd herpes virus succesvol bij melanoompatienten in Anthonie van Leeuwenhoek ziekenhuis.
- Biomarkers - DNA mutaties en receptorenexpressie - zijn bepalend voor succes van immuuntherapie bij melanomen al lijkt algemene dendritische celtherapie als immuuntherapie ook zinvol
- Nivolumab als enige behandeling geeft 34 procent 5 jaars overleving bij zwaar voorbehandelde melanoompatienten
- Immuuntherapie met het RIGVIR virus bij operabele melanomen met stadia IA t/m IIC geeft 35 procent meer overlevingen op 3 tot 5 jaar in vergelijking met standaard behandeling copy 1
- Immuuntherapie met TIL - tumor infiltrating lymfocyten zorgt bij een kwart van de deelnemers voor jarenlange ziektevrije tijd bij patienten met uitgezaaide melanomen copy 1
- Dendritische celtherapie gecombineerd met hyperthermie verdubbelt overleving (van 6 tot 13 maanden) van patienten met vergevorderde melanomen.
- Vaccinatie samen met interleukon-2 (IL-2) verlengt significant ziektevrije tijd van melanoompatienten in vergelijkiing met alleen interleukon 2.
- Immunotherapie - na chemo lijkt succesvol bij melanomen graad IV.
- Immuuntherapie Onderzoekers aan de universiteit van Virginia melden positieve resultaten uit een kleinschalige (26 deelnemers) gerandomiseerde phase II studie naar de effecten van een vaccin met multi-peptides
- Nederlandse ziekenhuizen die ipilimumab (Yervoy) of vemurafenib (Zelboraf) mogen voorschrijven voor uitgezaaide melanoom
- Immuuntherapie met Interferon Alpha 2B bij patienten met melanomen geeft ten opzichte van een wait and see beleid op een meetpunt van 3,8 jaar een significant verschil in mediane ziektevrije overlevingstijd van 18%. Aldus gerandomiseerde fase III studie
- Immuuntherapie: autovaccinatie (van eigen kankercellen wordt een vaccin gemaakt) bij melanoompatienten zorgt bij 50 procent van deelnemende patienten voor langjarige remissies. Vooral melanoompatienten met MELOE-1 lijken gevoelig voor deze aanpak
- Immuuntherapie: patient met vergevorderde melanoomkanker geneest doordat gekloonde cellen van eigen afweercellen als vaccin zijn teruggebracht in zjin lichaam.
- Monoclonaal middel werkt uitstekend als vaccinatie en immuunstimulerend middel bij muizen met melanoomkanker.
- Immuuntherapie en melanomen: Antibody therapie kan het positieve effect van een vaccin vergroten blijkt uit kleinschalige studie met 9 uitbehandelde eierstokkankerpatiënten en melanoompatiënten.
- Immuuntherapie bij melanomen: een overzicht van recente ontwikkelingen en belangrijke studies



Plaats een reactie ...
Reageer op "Pembrolizumab superieur in ziektevrije tijd, overall overleving en met significant minder bijwerkingen dan Ipilimumab bij inoperabele gevorderde melanomen."