Aan dit artikel is vele uren gewerkt. Opzoeken, vertalen, op de website plaatsen enz. Als u ons wilt ondersteunen dan kan dat via een al of niet anonieme donatie. Elk bedrag is welkom hoe klein ook. Klik hier als u ons wilt helpen kanker-actueel online te houden Wij zijn een ANBI organisatie en dus is uw donatie in principe aftrekbaar voor de belasting
4 augustus 2014: onderaan het ik het abstract toegevoegd van deze studie waarvan het volledige studierapport gratis is in te zien: Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease? met interessante referentielijst. Ook deze studie is mooi voorbeeld van een medicine dat ingrijpt op basis van bepaalde DNA mutaties en receptorenexpressie. Onder de grafiek staat meer informatie over vedolizumab bij ziekte van Crohn
22 augustus 2013: Bron: NEJM
Vedoluzimab bljikt uitstekend te werken als preventie en behandeling van ontstekingen in de darmen, ziekte van Crohn en bij colitis ulcerosa. Dit blijkt uit twee grote vergelijkende placebo gecontroleerde studies bij totaal ruim 2000 patiënten uitgevoerd in 39 landen. De verschillen waren statistisch significant.
Studieresultaten:
De onderzoekers hebben twee gerandomiseerde, dubbelblinde, placebo gecontroleerde studies van vedolizumab bij patiënten met een actieve ziekte met elkaar vergeleken. Als aanvangsbehandeling ontvingen 374 patiënten (cohort 1) vedolizumab (een dosis van 300 mg) of intraveneus een placebo in de weken 0 en 2. 521 patiënten (cohort 2) kregen een open-label vedolizumab in de weken 0 en 2. In week 6 werden de resultaten geevalueerd.
Als onderhoudsbehandeling kregen alle patiënten die goed hadden gereageerd op vedolizumab vervolgens willekeurig ingedeeld vedolizumab elke 8 of 4 weken of schakelden over op placebo gedurende 52 weken. Een respons werd gedefinieerd als een vermindering van de scores zoals die zijn vastgelegd in de Mayo Clinic (range, 0 tot 12, met hogere scores die meer actieve ziekte betekenen) van ten minste 3 punten en een daling van ten minste 30% van de uitgangswaarde, met een begeleidende afname in de rectale bloedingen subscore van minimaal 1 punt of een absolute rectale bloedingen subscore van 0 of 1.
Resultaten
Respons in week 6 was 47,1% onder patiënten in de vedolizumab groep en en 25,5% bij de patiënten uit de placebogroep (verschil met aanpassing voor stratificatiefactoren, 21,7 procentpunten; 95% betrouwbaarheidsinterval [BI], 11,6-31,7; P <0.001) . Na 1 jaar in week 52 bleken 41.8% van de patiënten die vedolizumab bleven ontvangen elke 8 weken en 44,8% van de patiënten die vedolizumab bleven ontvangen elke 4 weken in klinische remissie (Mayo Clinic score ≤ 2 en geen subscore> 1), vergeleken met 15,9 % van de patiënten die overschakelden naar placebo (gecorrigeerde verschil, 26,1 procentpunten voor vedolizumab elke 8 weken versus placebo ingeschakeld [95% BI, 14,9-37,2; P <0.001] en 29,1 procentpunten voor vedolizumab elke 4 weken versus placebo [95% BI, 17,9-40,4; P <0.001]). De frequentie van bijwerkingen was vergelijkbaar in de vedolizumab groep en de placebo groep.
Het volledige studieverslag: Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis is in te zien in de NEJM. Hier het abstract van deze studie
Vedolizumab was more effective than placebo as induction and maintenance therapy for ulcerative colitis
Original Article
Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis
N Engl J Med 2013; 369:699-710 August 22, 2013 DOI: 10.1056/NEJMoa1215734
Background
Gut-selective blockade of lymphocyte trafficking by vedolizumab may constitute effective treatment for ulcerative colitis.
Methods
We conducted two integrated randomized, double-blind, placebo-controlled trials of vedolizumab in patients with active disease. In the trial of induction therapy, 374 patients (cohort 1) received vedolizumab (at a dose of 300 mg) or placebo intravenously at weeks 0 and 2, and 521 patients (cohort 2) received open-label vedolizumab at weeks 0 and 2, with disease evaluation at week 6. In the trial of maintenance therapy, patients in either cohort who had a response to vedolizumab at week 6 were randomly assigned to continue receiving vedolizumab every 8 or 4 weeks or to switch to placebo for up to 52 weeks. A response was defined as a reduction in the Mayo Clinic score (range, 0 to 12, with higher scores indicating more active disease) of at least 3 points and a decrease of at least 30% from baseline, with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1.
Results
Response rates at week 6 were 47.1% and 25.5% among patients in the vedolizumab group and placebo group, respectively (difference with adjustment for stratification factors, 21.7 percentage points; 95% confidence interval , 11.6 to 31.7; P<0.001). At week 52, 41.8% of patients who continued to receive vedolizumab every 8 weeks and 44.8% of patients who continued to receive vedolizumab every 4 weeks were in clinical remission (Mayo Clinic score ≤2 and no subscore >1), as compared with 15.9% of patients who switched to placebo (adjusted difference, 26.1 percentage points for vedolizumab every 8 weeks vs. placebo [95% CI, 14.9 to 37.2; P<0.001] and 29.1 percentage points for vedolizumab every 4 weeks vs. placebo [95% CI, 17.9 to 40.4; P<0.001]). The frequency of adverse events was similar in the vedolizumab and placebo groups.
Conclusions
Vedolizumab was more effective than placebo as induction and maintenance therapy for ulcerative colitis. (Funded by Millennium Pharmaceuticals; GEMINI 1 ClinicalTrials.gov number, NCT00783718.)
Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease?
Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease?
Abstract
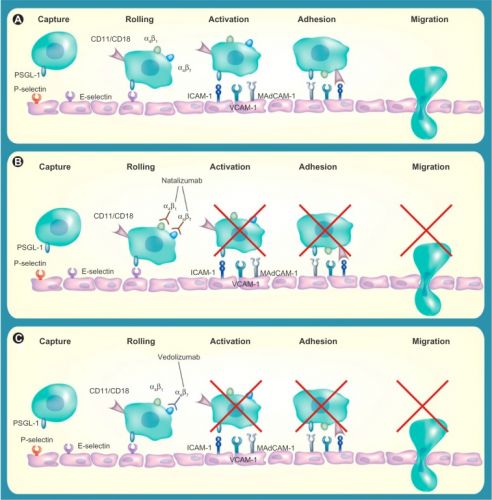
Inflammatory bowel disease (IBD) is an important cause of morbidity and mortality for millions of patients worldwide. Current treatment options include corticosteroids, 5-aminosalicylates, immunosuppressants, and TNFα antagonists. However, these are frequently ineffective in achieving sustained response and remission over time. At present, gastroenterologists lack safe and effective treatments if patients fail anti-TNF therapy. Vedolizumab is a promising new agent for IBD patients refractory to anti-TNF therapy. Vedolizumab is an integrin antagonist which is thought to act by reducing inflammation by selectively inhibiting leukocyte migration in the gut. Emerging evidence from clinical trials suggests a potential role for vedolizumab in both ulcerative colitis (UC) and Crohn’s disease (CD), particularly in patients who have previously failed biological therapy. The safety profile of vedolizumab appears reasonable, possibly because it has a “gut-selective” mode of action, with no reported cases of progressive multifocal leukoencephalopathy, a condition which has been linked to another integrin antagonist, natalizumab. This review discusses the available evidence for integrin antagonists and their potential role in the management of IBD.
References
Plaats een reactie ...
2 Reacties op "Vedolizumab voorkomt en geneest ontstekingen in darmen en lijkt uitstekend medicijn bij ziekte van Crohn"
Gerelateerde artikelen
- Moleculaire schakelaar verandert kankercellen in weer normale cellen en zou genezende aanpak van kanker kunnen betekenen copy 1
- Bispecifieke anti-lichaam REGN7075 in combinatie met Libtayo (cemiplimab) geeft veelbelovende resultaten bij patiënten met microsatelliet-stabiele darmkanker die ongevoelig zijn voor immuuntherapie
- 10-genentest voorspelt welke chemo het beste zou zijn voor patienten met darmkanker stadium II en III na operatie.
- Biomarkers zijn waardevol voor een gepersonaliseerde behandeling van darmkanker, bewijst de TAPUR-studie
- Overzicht van studies met medicijnen en behandelingen om tumoren met KRAS mutaties aan te pakken. Vooral combinatiebehandelingen zijn veelbelovend. copy 1 copy 1
- Temozolomide - temodal gevolgd door immunotherapie met combinatie van lage dosis ipilimumab plus nivolumab geeft hoopgevende resultaten bij patiënten met microsatellietstabiel en MGMT-gedempte uitgezaaide darmkanker copy 1
- NUC-3373, een thymidylate synthase remmer, geeft opmerkelijk goede resultaten zonder de bekende bijwerkingen bij zwaar voorbehandelde patiënten met uitgezaaide darmkanker
- Darmkankerpatienten stadium III met een instabiele MSI/dMMR leefden langer met een recidief dan met MSI/dMMR stabiel voordat immuuntherapie kon worden ingezet
- POLE mutatie: veel kankerpatienten met erfelijke vormen van kanker hebben naast een P1-ligand een POLE mutatie en reageren goed op immuuntherapie met anti-PD medicijnen - checkpointremmers als pembrolizumab en nivolumab copy 1
- Anti-EGFR anti body mix van medicijnen - SYM004 - geeft hoopvolle resultaten in fase I studie bij zwaar voorbehandelde darmkankerpatienten met RAS en EGFR positieve mutaties
- BRAF en EGFR mutaties gerichte medicijnencocktail zou ontduiking tegen het nieuwe succesvolle eiwit molecuul - PLX4032 - vemurafenib bij darmkanker opheffen, aldus Nederlandse onderzoekers
- Cimetidine - Tagamet-800 - een maagzuurremmer bewijst bij maagkanker en darmkanker een effectieve kankerremmer te zijn.
- Debulking plus op receptoren - mutaties gerichte aanpak voor in lever of andere organen uitgezaaide darmkanker wordt in fase I onderzocht in Nederland.
- DNA mutaties komen 3x zo vaak voor bij jonge mensen (gemiddeld 40 jaar) dan bij ouderen (gemiddeld 70 jaar) met darmkanker. copy 1
- Erbitux - Cetuximab faalt als aanvulling bij chemo (FOLFOX) voor darmkankerpatienten. Fase III studie is daarom afgebroken. Bij KRAS wild type werkt Erbitux wel.
- Klinische en genetische factoren voorspellen een eventuele respons op een behandeling bij patiënten met de ziekte van Crohn
- KRAS en NRAS mutatie bepaling cruciaal voor effectieve anti-EGFR behandeling bij uitgezaaide darmkanker met FOLFOX4 + Panitumumab
- Mifeprestone - (RU-486) - abortuspil stopt tumorgroei bij darmkankerpatienten met veruitgezaaide darmkanker en stopt ook groei van kanker bij andere vormen van kanker blijkt uit studies.
- Mutaties in andere genen - Nras, BRAF, PIK3CA en niet-functionele PTEN voorspellen uitkomsten en resistentie van anti-EGFR behandelingen bij KRAS wild type en toont de noodzaak van uitgebreidere biomarker analyse bij uitgezaaide darmkanker copy 1
- Nivolumab goedgekeurd door FDA voor MSI-H of dMMR uitgezaaide darmkanker na progressie van de ziekte gedurende of na chemo met FOLFOX en Folfirinox
- Onvansertib naast standaard chemotherapie krijgt van FDA versnelde goedkeuring na uitstekende resultaten bij darmkanker KRAS positief
- PIK3CA mutaties en vooral in PIK3CA exon 20 voorspellen gebrek aan respons voor anti-EGFR medicijnen bij uitgezaaide darmkanker met KRAS wild type
- Plaats van de tumor - rechts of links - heeft grote invloed op overall overleving en op keuze voor cetuximab of bevacizumab - avastin als behandeling voor uitgezaaide darmkanker KRAS wild type
- Panitumubab en Avastin bij darmkanker, een paar studies en een artikel in Nederlands Tijdschrift voor Geneeskunde over targeted therapie bij darmkankers.
- Raltritexed: Analyse van effect en kosten van irinotecan, oxaliplatin en raltitrexed al of niet in combinaties ten opzichte van 5-FU bij darmkanker.
- Regorafenib krijgt goedkeuring van de Europese Commissie voor het gebruik bij uitgezaaide darmkanker waarbij eerdere behandelingen falen.
- Uracil-tegafur (UFT) geeft zelfde resultaat bij uitgezaaide darmkanker als intraveneuze chemo (5-FU en leucovorin) maar veel minder bijwerkingen en veel gemakkelijker in te nemen
- Spectacolor studie bij darmkanker toont aan dat receptoren- en DNA onderzoek cruciaal is voor nieuwe op te zetten klinische studies en een goede optimale gepersonaliseerde behandeling bij darmkanker copy 1
- Sotorasib (AMG 510) geeft bij patienten met KRAS G12C mutatie bij patienten met zwaar voorbehandelde darmkanker en longkanker met KRAS pos. alsnog uitstekende resultaten copy 1 copy 1
- Vedolizumab voorkomt en geneest ontstekingen in darmen en lijkt uitstekend medicijn bij ziekte van Crohn
- Vinorelbine lijkt voor darmkanker met BRAF en KRAS mutatie een effectief medicijn aldus Rene Bernards na nieuwe studieresultaten uit basket studies
- Xilonix - MAPp1 zorgt voor stabiele ziekte (bij 53 procent) bij zwaar voorbehandelde darmkankerpatienten stadium 4 met een mediane overall overleving van 4.2 maanden vs 11.5 maanden in vergelijking met placebo
- Personalised medicin en gerichte aanpak - targeted therapy - op veel voorkomende receptoren en genmutaties bij vormen van darmkanker bij elkaar gezet in een overzicht



Ik wil het zeker een keer uitproberen. Tot nu toe reageer ik alleen goed op het medicijn entocort.