20 december 2016: Bron: World J Gastrointest Oncol. 2015 Apr 15; 7(4): 17–29.
Wanneer kankerpatiënten in een gevorderd stadium van hun ziekte verkeren krijgen ze bijna altijd te maken met sterke vermagering, ook wel cahexie genoemd. Ook chemokuren en nieuwe medicijn combinaties binnen personalised medicine veroorzaken vaak sterke vermagering. Uit de literatuur blijkt dat 20 procent ook vroegtijdig overlijdt mede door die sterke vermagering. De laatste jaren echter zijn er wel behandelingen gevonden die die vermagering tegengaan. Met als effect dat de kwaliteit van leven verbetert en het ook invloed heeft op de overall overleving.
In de literatuurlijsten van arts-bioloog drs. Engelbert Valstar staan ook per kankersoort of naast chemo studies vermeld van niet-toxische voedingsstoffen die vermagering tegengaan. Maar ook in de reguliere oncologie zijn er inmiddels wel synthethische stoffen / medicijnen die die vermagering kunnen tegengaan.
In dit studierapport: Cancer cachexia, mechanism and treatment worden een aantal behandelingen / middelen tegen cahexie beschreven. Zie o.a. de groen gemaakte sleutelwoorden.
Tekst gaat verder onder foto.

Bron foto: Medical facts:
http://www.medicalfacts.nl/2013/08/28/maastrichts-onderzoek-biedt-perspectief-voor-longkankerpatienten-met-spiermassaverlies/
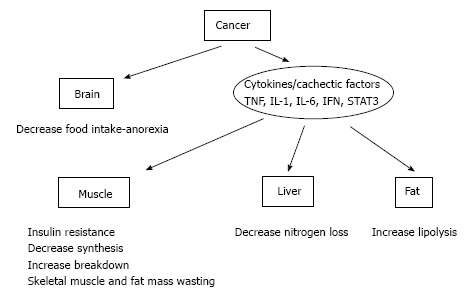
In bovenstaande grafiek wordt aangegeven hoe dat proces van vermagering precies werkt. Hoe de verschillende behandelingen, medicijnen en niet-toxische stoffen werken en ook met verwijzing naar studies met die aanpak is te zien in onderstaande tabel. Deze worden ook besproken in het studierapport. Hoewel het voor leken wel ingewikkeld is lijkt mij. Maar u zou het altijd eens voor kunnen leggen aan uw behandelend arts.
Een ander Nederlands onderzoek is ook interessant om te lezen hoe vermagering plaatsvindt bij loingkankerpatiënten en wat daar tegen te doen: Maastrichts onderzoek biedt perspectief voor longkankerpatiënten met spiermassaverlies
Tekst gaat verder onder grafiek
Table 1
| Treatment |
Description |
Physiologic benefit |
Possible mechanism |
Ref. |
| Megestrol acetate |
Active progesterone derivative |
Improves appetite, caloric intake, nutritional status, quality of life |
Unknown; possible neuropeptide Y release |
[80-92] |
| Medroxyprogesterone |
Active progesterone derivative |
Improves appetite, food intake Weight stabilization |
Decreases serotonin, IL-1, IL-6, TNF-α |
[93-96] |
| Ghrelin |
Gastric peptide hormone |
Improves lean + total body mass, hand grip, cardiac function (CHF cachexia only) |
Growth hormone receptor secretagogue |
|
| Delta-9-tetrahydrocannabinol |
Cannabinoid |
MIXED May improve food intake, weight gain |
Possible endorphin receptor activation, Inhibition of prostaglandin, IL-1 |
[85,106-110] |
| Melanocortin antagonists |
Adrenocorticotropic hormone antagonist |
UNTESTED; prevention of anorexia, loss of lean body mass or basal energy (animal only) |
Neuropeptide Y alteration or melanocortin-4 receptor antagonism |
[112,113] |
| Thalidomide |
Immunomodulatory |
Limits weight and lean body mass loss |
Decreases TNF-α, pro-inflammatory cytokines, nuclear factor kappa B, cyclooxygenase 2, angiogenesis |
[124-126] |
| Etanercept |
Immunomodulatory |
Limits fatigue; improves adjuvant therapy adherence |
Decreases TNF effect |
|
| Eicosapentaenoic acid/omega-3-fatty acids |
Lipid |
MIXED; may improve weight, appetite, quality of life |
Decreases pro-inflammatory cytokines, proteolysis inducing factor |
[129,130,133,137,140-142,146-152] |
| Rikkun-shito |
Herbal Japanese medicine |
Improves median survival with gemcitabine (pancreatic cancer); improves anorexia, GI dysmotility, muscle wasting, anxiety |
Unknown |
[154,155] |
| Corticosteroids |
Immunomodulatory |
Improves appetite and quality of life |
Various mechanisms |
[156,157] |
| Formoterol |
β2-adrenergic agonist |
UNTESTED |
Protein and muscle degradation antagonism |
|
| Erythropoetin |
Glycoprotein hormone |
Improves patient’s metabolic and exercise capacity |
Decreases production of IL-6 |
[171-173] |
| ACE inhibitors |
Heart medications |
Reduce wasting of muscle mass |
Inhibit TNF-α production |
|
| β-blockers |
Heart medications |
Preserved body weight, and lean and fat mass, and improved the quality of life |
Normalized Akt phosphorylation |
|
Combination therapy with diet modification and/or exercise has been added to novel pharmaceutical agents, such as Megestrol acetate, medroxyprogesterone, ghrelin, omega-3-fatty acid among others. These agents are reported to have improved survival rates as well as quality of life. In this review, we will discuss the emerging understanding of the mechanisms of cancer cachexia, the current treatment options including multidisciplinary combination therapies, as well an update on new and ongoing clinical trials.
Cancer cachexia, mechanism and treatment
This article has been
cited by other articles in PMC.
Abstract
It is estimated that half of all patients with cancer eventually develop a syndrome of cachexia, with anorexia and a progressive loss of adipose tissue and skeletal muscle mass. Cancer cachexia is characterized by systemic inflammation, negative protein and energy balance, and an involuntary loss of lean body mass. It is an insidious syndrome that not only has a dramatic impact on patient quality of life, but also is associated with poor responses to chemotherapy and decreased survival. Cachexia is still largely an underestimated and untreated condition, despite the fact that multiple mechanisms are reported to be involved in its development, with a number of cytokines postulated to play a role in the etiology of the persistent catabolic state. Existing therapies for cachexia, including orexigenic appetite stimulants, focus on palliation of symptoms and reduction of the distress of patients and families rather than prolongation of life. Recent therapies for the cachectic syndrome involve a multidisciplinary approach. Combination therapy with diet modification and/or exercise has been added to novel pharmaceutical agents, such as Megestrol acetate, medroxyprogesterone, ghrelin, omega-3-fatty acid among others. These agents are reported to have improved survival rates as well as quality of life. In this review, we will discuss the emerging understanding of the mechanisms of cancer cachexia, the current treatment options including multidisciplinary combination therapies, as well an update on new and ongoing clinical trials.
References
1.
Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. [PubMed]2.
Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99. [PubMed]3.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [PubMed]4.
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. [PubMed]5.
Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. [PubMed]6.
Congleton J. The pulmonary cachexia syndrome: aspects of energy balance. Proc Nutr Soc. 1999;58:321–328. [PubMed]7.
von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–252. [PubMed]8.
Giordano A, Calvani M, Petillo O, Carteni’ M, Melone MR, Peluso G. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem. 2003;90:170–186. [PubMed]9.
Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO, Engstrom PF, Ezdinli EZ, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. [PubMed]10.
Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–269. [PubMed]11.
Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11:400–407. [PubMed]12. Warren S. The immediate cause of death in cancer. Am J Med Sci. 1932;184:610–613.
13.
Skipworth RJ, Stewart GD, Dejong CH, Preston T, Fearon KC. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr. 2007;26:667–676. [PubMed]14.
Hopkinson JB, Wright DN, McDonald JW, Corner JL. The prevalence of concern about weight loss and change in eating habits in people with advanced cancer. J Pain Symptom Manage. 2006;32:322–331. [PubMed]15.
von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. [PMC free article] [PubMed]16.
Diffee GM, Kalfas K, Al-Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am J Physiol-Cell Ph. 2002;283:C1376–C1382. [PubMed]17.
Tijerina AJ. The biochemical basis of metabolism in cancer cachexia. Dimens Crit Care Nurs. 2004;23:237–243. [PubMed]18.
Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. [PubMed]19.
Argilés JM. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S39–S50. [PubMed]20.
Mantovani G, Madeddu C. Cancer cachexia: medical management. Support Care Cancer. 2010;18:1–9. [PubMed]21.
Springer J, von Haehling S, Anker SD. The need for a standardized definition for cachexia in chronic illness. Nat Clin Pract Endoc. 2006;2:416–417. [PubMed]22.
Lainscak M, Filippatos GS, Gheorghiade M, Fonarow GC, Anker SD. Cachexia: Common, deadly, with an urgent need for precise definition and new therapies. Am J Cardiol. 2008;101:8E–10E. [PubMed]23.
Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543–560. [PubMed]24.
Argilés JM, Busquets S, Toledo M, López-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care. 2009;3:263–268. [PubMed]25.
MacDonald N, Easson AM, Mazurak VC, Dunn GP, Baracos VE. Understanding and managing cancer cachexia. J Am Coll Surg. 2003;197:143–161. [PubMed]26.
Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, Kaasa S, Fearon K, Strasser F. Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol. 2011;80:114–144. [PubMed]27.
Deans DA, Tan BH, Wigmore SJ, Ross JA, de Beaux AC, Paterson-Brown S, Fearon KC. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer. 2009;100:63–69. [PMC free article] [PubMed]28.
Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. 1999;23:584–588. [PubMed]29.
Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. [PubMed]30.
Staal-van den Brekel AJ, Dentener MA, Schols AM, Buurman WA, Wouters EF. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol. 1995;13:2600–2605. [PubMed]31.
Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer. 1996;73:1560–1562. [PMC free article] [PubMed]32.
Blay JY, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, Mercatello A, Ravault A, Tourani JM, Moskovtchenko JF. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992;52:3317–3322. [PubMed]33.
Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. [PubMed]34.
O'Gorman P, McMillan DC, McArdle CS. Impact of weight loss, appetite, and the inflammatory response on quality of life in gastrointestinal cancer patients. Nutr Cancer. 1998;32:76–80. [PubMed]35.
Barber MD, Ross JA, Fearon KC. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35:106–110. [PubMed]36.
Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–910. [PubMed]37.
Barber MD, Fearon KC, McMillan DC, Slater C, Ross JA, Preston T. Liver export protein synthetic rates are increased by oral meal feeding in weight-losing cancer patients. Am J Physiol Endocrinol Metab. 2000;279:E707–E714. [PubMed]38.
Ross JA, Fearon KC. Eicosanoid-dependent cancer cachexia and wasting. Curr Opin Clin Nutr Metab Care. 2002;5:241–248. [PubMed]39.
Tisdale MJ. The ‘cancer cachectic factor’ Support Care Cancer. 2003;11:73–78. [PubMed]40.
Baracos VE, Mazurak VC, Ma DW. n-3 Polyunsaturated fatty acids throughout the cancer trajectory: influence on disease incidence, progression, response to therapy and cancer-associated cachexia. Nutr Res Rev. 2004;17:177–192. [PubMed]41.
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. [PubMed]42.
Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391:1548–1554. [PubMed]43.
Murphy KT, Chee A, Gleeson BG, Naim T, Swiderski K, Koopman R, Lynch GS. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am J Physiol Regul Integr Comp Physiol. 2011;301:R716–R726. [PubMed]44.
Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. [PubMed]45.
Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–279. [PubMed]46.
Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38:168–176. [PMC free article] [PubMed]47.
Haslett PA. Anticytokine approaches to the treatment of anorexia and cachexia. Semin Oncol. 1998;25:53–57. [PubMed]48.
Mantovani G, Macciò A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine activity in cancer-related anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate. Semin Oncol. 1998;25:45–52. [PubMed]49.
Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. [PubMed]50.
Plata-Salamán CR. Immunoregulators in the nervous system. Neurosci Biobehav Rev. 1991;15:185–215. [PubMed]51.
Plata-Salamán CR. Anorexia during acute and chronic disease. Nutrition. 1996;12:69–78. [PubMed]52.
Moldawer LL, Copeland EM. Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer. 1997;79:1828–1839. [PubMed]53.
Banks WA. Anorectic effects of circulating cytokines: role of the vascular blood-brain barrier. Nutrition. 2001;17:434–437. [PubMed]54.
Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello CA. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Invest. 1989;84:228–235. [PMC free article] [PubMed]55.
Torelli GF, Meguid MM, Moldawer LL, Edwards CK, Kim HJ, Carter JL, Laviano A, Rossi Fanelli F. Use of recombinant human soluble TNF receptor in anorectic tumor-bearing rats. Am J Physiol. 1999;277:R850–R855. [PubMed]56.
Yeh SS, Schuster MW. Geriatric cachexia: the role of cytokines. Am J Clin Nutr. 1999;70:183–197. [PubMed]57.
Laviano A, Meguid MM, Yang ZJ, Gleason JR, Cangiano C, Rossi Fanelli F. Cracking the riddle of cancer anorexia. Nutrition. 1996;12:706–710. [PubMed]58.
Picton SV. Aspects of altered metabolism in children with cancer. Int J Cancer Suppl. 1998;11:62–64. [PubMed]59.
Albrecht JT, Canada TW. Cachexia and anorexia in malignancy. Hematol Oncol Clin North Am. 1996;10:791–800. [PubMed]60.
Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. [PubMed]61.
Barber MD, Fearon KC, Tisdale MJ, McMillan DC, Ross JA. Effect of a fish oil-enriched nutritional supplement on metabolic mediators in patients with pancreatic cancer cachexia. Nutr Cancer. 2001;40:118–124. [PubMed]62.
Socher SH, Martinez D, Craig JB, Kuhn JG, Oliff A. Tumor necrosis factor not detectable in patients with clinical cancer cachexia. J Natl Cancer Inst. 1988;80:595–598. [PubMed]63.
Falconer JS, Ross JA, Fearon KC, Hawkins RA, O’Riordain MG, Carter DC. Effect of eicosapentaenoic acid and other fatty acids on the growth in vitro of human pancreatic cancer cell lines. Br J Cancer. 1994;69:826–832. [PMC free article] [PubMed]64.
Spitzner M, Ebner R, Wolff HA, Ghadimi BM, Wienands J, Grade M. STAT3: A Novel Molecular Mediator of Resistance to Chemoradiotherapy. Cancers (Basel) 2014;6:1986–2011. [PMC free article] [PubMed]65.
Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410–E421. [PMC free article] [PubMed]66.
Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:601434. [PMC free article] [PubMed]67.
Argilés JM, Moore-Carrasco R, Fuster G, Busquets S, López-Soriano FJ. Cancer cachexia: the molecular mechanisms. Int J Biochem Cell Biol. 2003;35:405–409. [PubMed]68.
Moley JF, Aamodt R, Rumble W, Kaye W, Norton JA. Body cell mass in cancer-bearing and anorexic patients. JPEN J Parenter Enteral Nutr. 1987;11:219–222. [PubMed]69.
Dworzak F, Ferrari P, Gavazzi C, Maiorana C, Bozzetti F. Effects of cachexia due to cancer on whole body and skeletal muscle protein turnover. Cancer. 1998;82:42–48. [PubMed]70.
Tisdale MJ. Loss of skeletal muscle in cancer: biochemical mechanisms. Front Biosci. 2001;6:D164–D174. [PubMed]71.
Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. [PMC free article] [PubMed]72.
Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. 2013;4:95–109. [PMC free article] [PubMed]73.
Fredrix EW, Soeters PB, Wouters EF, Deerenberg IM, von Meyenfeldt MF, Saris WH. Effect of different tumor types on resting energy expenditure. Cancer Res. 1991;51:6138–6141. [PubMed]74.
Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994;219:325–331. [PMC free article] [PubMed]75.
Rigaud D, Hassid J, Meulemans A, Poupard AT, Boulier A. A paradoxical increase in resting energy expenditure in malnourished patients near death: the king penguin syndrome. Am J Clin Nutr. 2000;72:355–360. [PubMed]76.
Shellock FG, Riedinger MS, Fishbein MC. Brown adipose tissue in cancer patients: possible cause of cancer-induced cachexia. J Cancer Res Clin Oncol. 1986;111:82–85. [PubMed]77.
Qualliotine-Mann D, Agwu DE, Ellenburg MD, McCall CE, McPhail LC. Phosphatidic acid and diacylglycerol synergize in a cell-free system for activation of NADPH oxidase from human neutrophils. J Biol Chem. 1993;268:23843–23849. [PubMed]78.
Bing C, Brown M, King P, Collins P, Tisdale MJ, Williams G. Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res. 2000;60:2405–2410. [PubMed]79.
Collins P, Bing C, McCulloch P, Williams G. Muscle UCP-3 mRNA levels are elevated in weight loss associated with gastrointestinal adenocarcinoma in humans. Br J Cancer. 2002;86:372–375. [PMC free article] [PubMed]80.
Loprinzi CL, Michalak JC, Schaid DJ, Mailliard JA, Athmann LM, Goldberg RM, Tschetter LK, Hatfield AK, Morton RF. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol. 1993;11:762–767. [PubMed]81.
Bruera E, Macmillan K, Kuehn N, Hanson J, MacDonald RN. A controlled trial of megestrol acetate on appetite, caloric intake, nutritional status, and other symptoms in patients with advanced cancer. Cancer. 1990;66:1279–1282. [PubMed]82.
Neri B, Garosi VL, Intini C. Effect of medroxyprogesterone acetate on the quality of life of the oncologic patient: a multicentric cooperative study. Anticancer Drugs. 1997;8:459–465. [PubMed]83.
Nelson KA. The cancer anorexia-cachexia syndrome. Semin Oncol. 2000;27:64–68. [PubMed]84.
Gagnon B, Bruera E. A review of the drug treatment of cachexia associated with cancer. Drugs. 1998;55:675–688. [PubMed]85.
Argilés JM, Meijsing SH, Pallarés-Trujillo J, Guirao X, López-Soriano FJ. Cancer cachexia: a therapeutic approach. Med Res Rev. 2001;21:83–101. [PubMed]86.
Feliu J, González-Barón M, Berrocal A, Ordóñez A, Barón-Saura JM. Treatment of cancer anorexia with megestrol acetate: which is the optimal dose? J Natl Cancer Inst. 1991;83:449–450. [PubMed]87.
Loprinzi CL, Ellison NM, Schaid DJ, Krook JE, Athmann LM, Dose AM, Mailliard JA, Johnson PS, Ebbert LP, Geeraerts LH. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst. 1990;82:1127–1132. [PubMed]88.
Tchekmedyian NS, Hickman M, Siau J, Greco FA, Keller J, Browder H, Aisner J. Megestrol acetate in cancer anorexia and weight loss. Cancer. 1992;69:1268–1274. [PubMed]89.
Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. Body-composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol. 1993;11:152–154. [PubMed]90.
Rowland KM, Loprinzi CL, Shaw EG, Maksymiuk AW, Kuross SA, Jung SH, Kugler JW, Tschetter LK, Ghosh C, Schaefer PL, et al. Randomized double-blind placebo-controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive-stage small-cell lung cancer: a North Central Cancer Treatment Group study. J Clin Oncol. 1996;14:135–141. [PubMed]91.
Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;3:CD004310. [PubMed]92.
McCarthy HD, Crowder RE, Dryden S, Williams G. Megestrol acetate stimulates food and water intake in the rat: effects on regional hypothalamic neuropeptide Y concentrations. Eur J Pharmacol. 1994;265:99–102. [PubMed]93.
Mantovani G, Macciò A, Massa E, Madeddu C. Managing cancer-related anorexia/cachexia. Drugs. 2001;61:499–514. [PubMed]94.
Maltoni M, Nanni O, Scarpi E, Rossi D, Serra P, Amadori D. High-dose progestins for the treatment of cancer anorexia-cachexia syndrome: a systematic review of randomised clinical trials. Ann Oncol. 2001;12:289–300. [PubMed]95.
Mantovani G, Macciò A, Esu S, Lai P, Santona MC, Massa E, Dessì D, Melis GB, Del Giacco GS. Medroxyprogesterone acetate reduces the in vitro production of cytokines and serotonin involved in anorexia/cachexia and emesis by peripheral blood mononuclear cells of cancer patients. Eur J Cancer. 1997;33:602–607. [PubMed]96.
Costa AM, Spence KT, Plata-Salamán CR, ffrench-Mullen JM. Residual Ca2+ channel current modulation by megestrol acetate via a G-protein alpha s-subunit in rat hypothalamic neurones. J Physiol. 1995;487(Pt 2):291–303. [PMC free article] [PubMed]97.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. [PubMed]98.
Cheung CK, Wu JC. Role of ghrelin in the pathophysiology of gastrointestinal disease. Gut Liver. 2013;7:505–512. [PMC free article] [PubMed]99.
Hanada T, Toshinai K, Date Y, Kajimura N, Tsukada T, Hayashi Y, Kangawa K, Nakazato M. Upregulation of ghrelin expression in cachectic nude mice bearing human melanoma cells. Metabolism. 2004;53:84–88. [PubMed]100.
Kerem M, Ferahkose Z, Yilmaz UT, Pasaoglu H, Ofluoglu E, Bedirli A, Salman B, Sahin TT, Akin M. Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol. 2008;14:3633–3641. [PMC free article] [PubMed]101.
Takahashi M, Terashima M, Takagane A, Oyama K, Fujiwara H, Wakabayashi G. Ghrelin and leptin levels in cachectic patients with cancer of the digestive organs. Int J Clin Oncol. 2009;14:315–320. [PubMed]102.
Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778. [PubMed]103.
Karapanagiotou EM, Polyzos A, Dilana KD, Gratsias I, Boura P, Gkiozos I, Syrigos KN. Increased serum levels of ghrelin at diagnosis mediate body weight loss in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2009;66:393–398. [PubMed]104.
Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–2926. [PubMed]105.
Nagaya N, Kojima M, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med. 2006;45:127–134. [PubMed]106.
Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52:72–91. [PubMed]107.
Gorter RW. Cancer cachexia and cannabinoids. Forsch Komplementarmed. 1999;6 Suppl 3:21–22. [PubMed]108.
Mitchelson F. Pharmacological agents affecting emesis. A review (Part II) Drugs. 1992;43:443–463. [PubMed]109.
Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, Pundaleeka S, Kardinal CG, Fitch TR, Krook JE, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567–573. [PubMed]110.
Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24:3394–3400. [PubMed]111.
Marks DL, Butler AA, Turner R, Brookhart G, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144:1513–1523. [PubMed]112.
Scarlett JM, Marks DL. The use of melanocortin antagonists in cachexia of chronic disease. Expert Opin Investig Drugs. 2005;14:1233–1239. [PubMed]113.
DeBoer MD, Marks DL. Therapy insight: Use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2:459–466. [PubMed]114.
Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987;50:555–563. [PubMed]115.
Langstein HN, Doherty GM, Fraker DL, Buresh CM, Norton JA. The roles of gamma-interferon and tumor necrosis factor alpha in an experimental rat model of cancer cachexia. Cancer Res. 1991;51:2302–2306. [PubMed]116.
Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. [PMC free article] [PubMed]117.
Costelli P, Carbó N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argilés JM, Baccino FM. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783–2789. [PMC free article] [PubMed]118.
Murray S, Schell K, McCarthy DO, Albertini MR. Tumor growth, weight loss and cytokines in SCID mice. Cancer Lett. 1997;111:111–115. [PubMed]119.
Matthys P, Heremans H, Opdenakker G, Billiau A. Anti-interferon-gamma antibody treatment, growth of Lewis lung tumours in mice and tumour-associated cachexia. Eur J Cancer. 1991;27:182–187. [PubMed]120.
Strassmann G, Kambayashi T. Inhibition of experimental cancer cachexia by anti-cytokine and anti-cytokine-receptor therapy. Cytokines Mol Ther. 1995;1:107–113. [PubMed]121.
Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7:427–434. [PubMed]122.
Inui A. Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59:4493–4501. [PubMed]123.
Yamamoto N, Kawamura I, Nishigaki F, Tsujimoto S, Naoe Y, Inami M, Elizabeth L, Manda T, Shimomura K. Effect of FR143430, a novel cytokine suppressive agent, on adenocarcinoma colon26-induced cachexia in mice. Anticancer Res. 1998;18:139–144. [PubMed]124.
Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703. [PMC free article] [PubMed]125.
Gordon JN, Goggin PM. Thalidomide and its derivatives: emerging from the wilderness. Postgrad Med J. 2003;79:127–132. [PMC free article] [PubMed]126.
Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. 2005;54:540–545. [PMC free article] [PubMed]127.
Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. [PubMed]128.
Tisdale MJ. Mechanism of lipid mobilization associated with cancer cachexia: interaction between the polyunsaturated fatty acid, eicosapentaenoic acid, and inhibitory guanine nucleotide-regulatory protein. Prostaglandins Leukot Essent Fatty Acids. 1993;48:105–109. [PubMed]129.
Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–891. [PubMed]130.
Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–1747. [PubMed]131.
Colomer R, Moreno-Nogueira JM, García-Luna PP, García-Peris P, García-de-Lorenzo A, Zarazaga A, Quecedo L, del Llano J, Usán L, Casimiro C. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007;97:823–831. [PubMed]132.
Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996–1002. [PMC free article] [PubMed]133.
Barber MD, Ross JA, Preston T, Shenkin A, Fearon KC. Fish oil-enriched nutritional supplement attenuates progression of the acute-phase response in weight-losing patients with advanced pancreatic cancer. J Nutr. 1999;129:1120–1125. [PubMed]134.
Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KC. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999;81:80–86. [PMC free article] [PubMed]135.
Barber MD, McMillan DC, Preston T, Ross JA, Fearon KC. Metabolic response to feeding in weight-losing pancreatic cancer patients and its modulation by a fish-oil-enriched nutritional supplement. Clin Sci (Lond) 2000;98:389–399. [PubMed]136.
Barber MD, Fearon KC. Tolerance and incorporation of a high-dose eicosapentaenoic acid diester emulsion by patients with pancreatic cancer cachexia. Lipids. 2001;36:347–351. [PubMed]137.
Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, Baracos V. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol. 2003;21:129–134. [PubMed]138.
Burns CP, Halabi S, Clamon GH, Hars V, Wagner BA, Hohl RJ, Lester E, Kirshner JJ, Vinciguerra V, Paskett E. Phase I clinical study of fish oil fatty acid capsules for patients with cancer cachexia: cancer and leukemia group B study 9473. Clin Cancer Res. 1999;5:3942–3947. [PubMed]139.
Burns CP, Halabi S, Clamon G, Kaplan E, Hohl RJ, Atkins JN, Schwartz MA, Wagner BA, Paskett E. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia. Cancer. 2004;101:370–378. [PubMed]140.
Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J, Barber MD, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–1486. [PMC free article] [PubMed]141.
Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998;82:395–402. [PubMed]142.
Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, Gagnon B, Novotny PJ, Mailliard JA, Bushey TI, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol. 2004;22:2469–2476. [PubMed]143.
Kenler AS, Swails WS, Driscoll DF, DeMichele SJ, Daley B, Babineau TJ, Peterson MB, Bistrian BR. Early enteral feeding in postsurgical cancer patients. Fish oil structured lipid-based polymeric formula versus a standard polymeric formula. Ann Surg. 1996;223:316–333. [PMC free article] [PubMed]144.
Swails WS, Kenler AS, Driscoll DF, DeMichele SJ, Babineau TJ, Utsunamiya T, Chavali S, Forse RA, Bistrian BR. Effect of a fish oil structured lipid-based diet on prostaglandin release from mononuclear cells in cancer patients after surgery. JPEN J Parenter Enteral Nutr. 1997;21:266–274. [PubMed]145.
Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, Fearon KC. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition. 1996;12:S27–S30. [PubMed]146.
Wigmore SJ, Barber MD, Ross JA, Tisdale MJ, Fearon KC. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer. 2000;36:177–184. [PubMed]147.
Dewey A, Baughan C, Dean T, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007;(1):CD004597. [PubMed]148.
Anker SD, Coats AJ. How to recover from renaissance? The significance of the results of recover, renaissance, renewal and attach. Int J Cardiol. 2002;86:123–130. [PubMed]149.
Zuijdgeest-Van Leeuwen SD, Dagnelie PC, Wattimena JL, Van den Berg JW, Van der Gaast A, Swart GR, Wilson JH. Eicosapentaenoic acid ethyl ester supplementation in cachectic cancer patients and healthy subjects: effects on lipolysis and lipid oxidation. Clin Nutr. 2000;19:417–423. [PubMed]150.
Mazzotta P, Jeney CM. Anorexia-cachexia syndrome: a systematic review of the role of dietary polyunsaturated Fatty acids in the management of symptoms, survival, and quality of life. J Pain Symptom Manage. 2009;37:1069–1077. [PubMed]151.
Persson C, Glimelius B, Rönnelid J, Nygren P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition. 2005;21:170–178. [PubMed]152.
Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, Murray GD. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–3407. [PubMed]153.
Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer. 2011;47:508–514. [PMC free article] [PubMed]154.
Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, Yada T, Maejima Y, Sedbazar U, Sakai T, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1:e23. [PMC free article] [PubMed]155.
Huang CF, Lin SS, Liao PH, Young SC, Yang CC. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol Immunol. 2008;5:23–31. [PMC free article] [PubMed]156.
Moertel CG, Schutt AJ, Reitemeier RJ, Hahn RG. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer. 1974;33:1607–1609. [PubMed]157.
Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, Rowland KM, Camoriano JK, Novotny PJ, Christensen BJ. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–3306. [PubMed]158.
Lai V, George J, Richey L, Kim HJ, Cannon T, Shores C, Couch M. Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck. 2008;30:67–74. [PubMed]159.
McMillan DC, Wigmore SJ, Fearon KC, O’Gorman P, Wright CE, McArdle CS. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer. 1999;79:495–500. [PMC free article] [PubMed]160.
Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59:14–20. [PubMed]161.
Lundholm K, Gelin J, Hyltander A, Lönnroth C, Sandström R, Svaninger G, Körner U, Gülich M, Kärrefors I, Norli B. Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer Res. 1994;54:5602–5606. [PubMed]162.
Reid J, Hughes CM, Murray LJ, Parsons C, Cantwell MM. Non-steroidal anti-inflammatory drugs for the treatment of cancer cachexia: a systematic review. Palliat Med. 2013;27:295–303. [PubMed]163.
Solheim TS, Fearon KC, Blum D, Kaasa S. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol. 2013;52:6–17. [PubMed]164.
Kim YS, Sainz RD. Beta-adrenergic agonists and hypertrophy of skeletal muscles. Life Sci. 1992;50:397–407. [PubMed]165.
Agbenyega ET, Wareham AC. Effect of clenbuterol on skeletal muscle atrophy in mice induced by the glucocorticoid dexamethasone. Comp Biochem Physiol Comp Physiol. 1992;102:141–145. [PubMed]166.
Rajab P, Fox J, Riaz S, Tomlinson D, Ball D, Greenhaff PL. Skeletal muscle myosin heavy chain isoforms and energy metabolism after clenbuterol treatment in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1076–R1081. [PubMed]167.
Hinkle RT, Hodge KM, Cody DB, Sheldon RJ, Kobilka BK, Isfort RJ. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve. 2002;25:729–734. [PubMed]168.
Yang YT, McElligott MA. Multiple actions of beta-adrenergic agonists on skeletal muscle and adipose tissue. Biochem J. 1989;261:1–10. [PMC free article] [PubMed]169.
Mersmann HJ. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci. 1998;76:160–172. [PubMed]170.
Busquets S, Figueras MT, Fuster G, Almendro V, Moore-Carrasco R, Ametller E, Argilés JM, López-Soriano FJ. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 2004;64:6725–6731. [PubMed]171.
Penna F, Busquets S, Toledo M, Pin F, Massa D, López-Soriano FJ, Costelli P, Argilés JM. Erythropoietin administration partially prevents adipose tissue loss in experimental cancer cachexia models. J Lipid Res. 2013;54:3045–3051. [PMC free article] [PubMed]172.
van Halteren HK, Bongaerts GP, Verhagen CA, Kamm YJ, Willems JL, Grutters GJ, Koopman JP, Wagener DJ. Recombinant human erythropoietin attenuates weight loss in a murine cancer cachexia model. J Cancer Res Clin Oncol. 2004;130:211–216. [PubMed]173.
Kanzaki M, Soda K, Gin PT, Kai T, Konishi F, Kawakami M. Erythropoietin attenuates cachectic events and decreases production of interleukin-6, a cachexia-inducing cytokine. Cytokine. 2005;32:234–239. [PubMed]174.
Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005;93:425–434. [PMC free article] [PubMed]175.
Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Pötsch M, von Websky K, et al. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J. 2014;35:932–941. [PMC free article] [PubMed]176.
de Gramont A, de Gramont A, Chibaudel B, Larsen AK, Tournigand C, André T. The evolution of adjuvant therapy in the treatment of early-stage colon cancer. Clin Colorectal Cancer. 2011;10:218–226. [PubMed]177.
Mantovani G, Macciò A, Madeddu C, Gramignano G, Lusso MR, Serpe R, Massa E, Astara G, Deiana L. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol Biomarkers Prev. 2006;15:1030–1034. [PubMed]178.
Kumar NB, Kazi A, Smith T, Crocker T, Yu D, Reich RR, Reddy K, Hastings S, Exterman M, Balducci L, et al. Cancer cachexia: traditional therapies and novel molecular mechanism-based approaches to treatment. Curr Treat Options Oncol. 2010;11:107–117. [PMC free article] [PubMed]179.
Couch M, Lai V, Cannon T, Guttridge D, Zanation A, George J, Hayes DN, Zeisel S, Shores C. Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck. 2007;29:401–411. [PubMed]180.
Lundholm K, Daneryd P, Bosaeus I, Körner U, Lindholm E. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: Effects on survival, metabolism, and function. Cancer. 2004;100:1967–1977. [PubMed]181.
Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:371–385. [PubMed]182.
Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. [PMC free article] [PubMed]183.
Toledo M, Busquets S, Sirisi S, Serpe R, Orpí M, Coutinho J, Martínez R, López-Soriano FJ, Argilés JM. Cancer cachexia: physical activity and muscle force in tumour-bearing rats. Oncol Rep. 2011;25:189–193. [PubMed]184.
Baltgalvis KA, Berger FG, Peña MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflugers Arch. 2009;457:989–1001. [PMC free article] [PubMed]185.
Weber MA, Krakowski-Roosen H, Schröder L, Kinscherf R, Krix M, Kopp-Schneider A, Essig M, Bachert P, Kauczor HU, Hildebrandt W. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009;48:116–124. [PubMed]186.
Aulino P, Berardi E, Cardillo VM, Rizzuto E, Perniconi B, Ramina C, Padula F, Spugnini EP, Baldi A, Faiola F, et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. [PMC free article] [PubMed]187. Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical practice guidelines on cancer cachexia in advanced cancer patients. Aachen: Department of Palliative Medicinen/European Palliative Care Research Collaborative; 2010.
188.
Ardies CM. Exercise, cachexia, and cancer therapy: a molecular rationale. Nutr Cancer. 2002;42:143–157. [PubMed]189.
al-Majid S, McCarthy DO. Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs. 2001;2:186–197. [PubMed]190.
Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, Oredalen E, Frantzen TL, Lesteberg I, Amundsen L, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16:1649–1657. [PMC free article] [PubMed]191.
Argilés JM, Busquets S, López-Soriano FJ, Costelli P, Penna F. Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle. 2012;3:73–76. [PMC free article] [PubMed]192.
Aagaard P. Making muscles “stronger”: exercise, nutrition, drugs. J Musculoskelet Neuronal Interact. 2004;4:165–174. [PubMed]193.
Bossola M, Muscaritoli M, Costelli P, Grieco G, Bonelli G, Pacelli F, Fanelli FR, Doglietto GB, Baccino FM. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237:384–389. [PMC free article] [PubMed]
Articles from World Journal of Gastrointestinal Oncology are provided here courtesy of Baishideng Publishing Group Inc
voeding, voedingsupplementen, cox-2 remmers, EPO, metabolisme, cahexie, vermagering, kanker, visolie, kampo, EPA, omega-3 vetzuren, cannabis olie, wietolie
Gerelateerde artikelen
- RadboudUMC begint met long read whole genome sequencing testen voor opsporen van genetische afwijkingen bij zeldzame aandoeningen
- Grote verschillen in kwaliteit van zorg voor kankerpatienten tussen Nederlandse ziekenhuizen blijkt uit recent rapport van het NFK - Nederlandse Federatie van Kankerpatiëntenorganisaties
- CIPD = chronische inflammatoire demyeliniserende polyradiculoneuropathie geeft het beste resultaat met maximaal drie lage doses IVIg = Intraveneus immunoglobuline
- Lipidenverlagende medicijnen (LLM) (statines) hebben gunstige invloed op overall overleving van kankerpatienten met borstkanker, darmkanker en melanomen.
- Olanzapine, een atypisch antipsychoticum, blijkt door onbekende oorzaak veroorzaakte misselijkheid en braken bijna volledig te voorkomen en weg te nemen bij kankerpatienten met gevorderde kanker
- Oudere mensen met kanker lopen groter risico op de bacterie Clostridium difficile (veroorzaakt diarree) en overlijden daaraan ook vaker dan mensen zonder kanker.
- Opereren zonder snijden met bv. TACE, RFA, Nanoknife, yttrium-90, cryoablatie enz.: doe een consult bij specialistisch team in Nederland voordat u naar het buitenland op zoek gaat.
- Alle abstracten van SGO 2019 (50th Annual Meeting of the Society of Gynecologic Oncology)
- Slechts 5 van de 16 medicijnen, waaronder anti-PD medicijnen, goedgekeurd door FDA voor gebruik bij vormen van spijsverteringskanker blijken daadwerkelijk effectief in overall overleving, kwaliteit van leven en kosteneffectief.
- Vermagering door kanker en behandelingen van kanker is steeds beter tegen te gaan en verbetert vaker kwaliteit van leven en overall overleving. Een overzicht van effectieve behandelingen
- FDG PET/MRI geeft vergelijkbare of zelfs betere resultaten vergeleken met een FDG PET/CT bij verschillende vormen van kanker aldus een meta-analyse van 29 studies
- Kanker bij kinderen heeft lang na genezing nog gevolgen. 40 procent krijgt een levensbedreigende ziekte in haar/zijn verdere leven.
- LDL + DHA (visolie) verpakt en ingebracht met nanodeeltjes doodt razendsnel 80 procent van levertumoren en spaart gewone cellen blijkt uit dierstudie
- Radioloog Dr. Jelle Barentsz geeft uitleg via videofilmpjes over nieuwste niet-invasieve behandelingen en de rol van de beeldvorming via mp-MRI bij o.a. prostaatkanker.
- Voorlichtingsfolder van het KWF over hoe uitzaaiingen ontstaan
- Overlevenden van kinderkanker lopen 5 keer zo groot risico op ernstige endocriene - hormonale - aandoeningen op latere leeftijd
- Remming van glucose opname in senescente cellen - verouderde slapende stamcellen - doet tumorcellen afsterven en kan kankerpatienten behoeden voor een recidief
- Gerichte behandelingen op basis van moleculaire tumor profielen van de individuele kankerpatient lijken de toekomst te zijn. Aldus eerste gerandomiseerde SHIVA studie
- Algemeen: Studies, ook fase III studies, met een negatief of geen resultaat worden zelden of nooit gepubliceerd.
- Algemeen: Nieuw ontdekte genen - Fanconi genen - zou ontstaan van erfelijke kanker verklaren, aldus Nederlandse onderzoekers.
- Door bedrijven gesponsorde studies geven significant vaker een rooskleuriger beeld van de resultaten dan onafhankelijk uitgevoerde studies
- Algemene artikelen die met kanker te maken hebben maar niet specifiek bij een vorm van kanker





Plaats een reactie ...
Reageer op "Vermagering door kanker en behandelingen van kanker is steeds beter tegen te gaan en verbetert vaker kwaliteit van leven en overall overleving. Een overzicht van effectieve behandelingen"