Helpt u ons aan 500 donateurs om kanker-actueel online te houden?
27 juli 2018: Bron: BMC Cancer,
- Received: 19 August 2017
- Accepted: 22 June 2018
- Published: 28 June 2018
Eribulin mesystate (HALAVEN ) (geregistreerd in Japan en Amerika als medicijn bij uitgezaaide borstkanker na minimaal twee chemokuren) blijkt als eerstelijns en tweedelijns bij gevorderde lokaal en / of uitgezaaide borstkanker uitstekend te werken. Uit een Japanse fase II studie zijn inmiddels 32 patiënten geëvalueerd en er werden uitstekende resultaten mee bereikt die minimaal gelijk waren aan chemo (paclitaxel en/of capecitabine - Xeloda) of eigenlijk beter bij patiënten met lokaal gevorderde en/of uitgezaaide borstkanker.(Tekst gaat verder onder foto)
 Bron foto is deze website
Bron foto is deze website
Halaven is a synthetic analogue of halichondrin b, isolated from the marine sponge Halichondria Okadai. Photo: courtesy of Eisai.
Studieresultaten:
43,8% van de 32 patiënten die op moment van de tussenevaluatie konder worden beoordeeld reageerden goed op dit medicijn, met een klinisch voordeel van 56,3% en een tumorcontrole van 78,1% (stabiele ziekte minimaal). De mediane ziekteprogressievrije overleving was 8,3 maanden. Vaak gemelde bijwerkingen waren neutropenie, haaruitval en perifere neuropathie. Uit de groep van 32 bereikten er drie een CR = complete remissie. Dat is opmerkelijk omdat er zelden uit deze groep van patiënten een complete remissie bereiken met chemo. Hier is dat dus 10 procent.
Hier respectievelijk de karakteristieken van de deelnemende vrouwen en daaronder een grafiek met de resultaten per patiënt:
Objective response rates for over all population and subgroups of patients
|
N |
Overall response rate (%) |
Clinical benefit rate (%) |
Median PFS (95%CI), months | |
|---|---|---|---|---|
|
Overall |
32 |
43.8 |
56.3 |
8.3 (7.1–9.4) |
|
Hormone receptor status |
||||
|
HR(−) |
12 |
33.3 |
41.7 |
5.5 (0–11.5) |
|
HR(+) |
20 |
50 |
65 |
8.8 (6.46–11.0) |
|
No. of prior chemotherapy |
||||
|
0 |
22 |
50 |
54.5 |
8.8 (7.4–10.1) |
|
1 |
10 |
30 |
70 |
8.3 (3.7–12.8) |
|
Metastatic site |
||||
|
Visceral |
23 |
43.5 |
60.9 |
8.3 (7.2–9.3) |
|
Non-visceral |
9 |
44.4 |
44.4 |
9.0 (4.2–13.8) |
|
Dose reduction during treatment |
||||
|
No reduction |
18 |
33.3 |
50 |
8.8 (3.9–13.6) |
|
Reduction |
14 |
57.1 |
64.3 |
8.3 (7.8–8.7) |
|
Hormone receptor status |
||||
|
Triple negative |
11 |
36.4 |
36.4 |
5.5 (1.3–9.7) |
|
Other |
21 |
47.6 |
71.4 |
8.8 (7.0–10.5) |
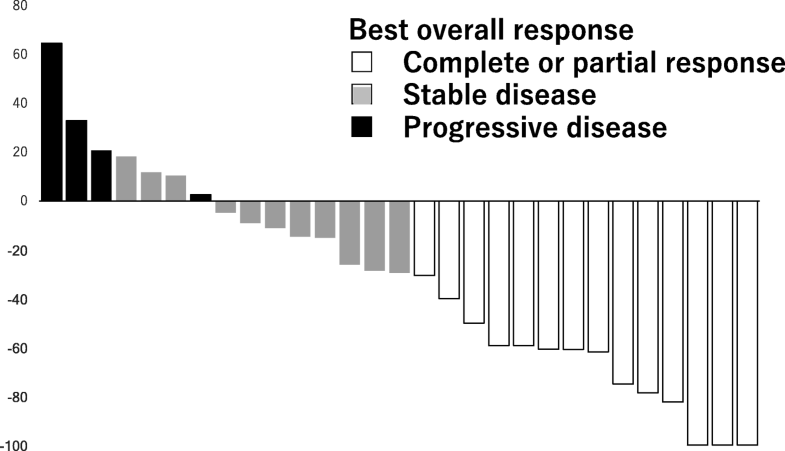
Hier een grafiek van de resultaten uit de studie:
Efficacy outcomes
|
No. of patients |
% |
95%CI | |
|---|---|---|---|
|
All assessable patients |
32 |
||
|
CR |
3 |
9.4 |
|
|
PR |
11 |
34.4 |
|
|
SD |
11 |
34.4 |
|
|
PD |
4 |
12.5 |
|
|
NE |
3 |
9.4 |
|
|
Overall response (CR + PR) |
14 |
43.8 |
(26.5–61.0) |
|
Clinical benefit rate (CR + PR + SD≧6 months) |
18 |
56.3 |
(39.0–73.5) |
|
Tumor control rate (CR + PR + SD) |
25 |
78.1 |
(63.8–92.5) |
Waterfall graphs of percentage change in the total sum of target lesion diameters from baseline to postbaseline nadir (RECIST v1.1)
Het volldige studierapport: Phase II trial of eribulin mesylate as a first- or second-line treatment for locally advanced or metastatic breast cancer: a multicenter, single-arm trial met nog veel meer duidelijke grafieken is gratis in te zien.
This trial was retrospectively registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (ID number: UMIN000010334).
Hier het abstract van de studie:
Eribulin may be another option for first-line chemotherapeutic regimens for metastatic breast cancer.
Phase II trial of eribulin mesylate as a first- or second-line treatment for locally advanced or metastatic breast cancer: a multicenter, single-arm trial
- Tetsu HayashidaEmail authorView ORCID ID profile,
- Hiromitsu Jinno,
- Katsuaki Mori,
- Hiroki Sato,
- Akira Matsui,
- Takashi Sakurai,
- Hiroaki Hattori,
- Shin Takayama,
- Masahiro Wada,
- Maiko Takahashi,
- Hirohito Seki,
- Tomoko Seki,
- Aiko Nagayama,
- Akiko Matsumoto and
- Yuko Kitagawa
- Received: 19 August 2017
- Accepted: 22 June 2018
- Published: 28 June 2018
Background
Eribulin mesylate is currently indicated as a sequential monotherapy to be administered after two chemotherapeutic regimens, including anthracycline and taxane treatments, for treatment of metastatic breast cancer. This open-label, multicenter phase II study was designed to evaluate the efficacy and safety of eribulin as a first- or second-line treatment for patients with metastatic breast cancer.
Methods
The primary objective was to determine the overall response rate. Secondary objectives were to evaluate progression-free survival and the safety profile. Patients were scheduled to receive eribulin mesylate 1.4 mg/m2 intravenously on days 1 and 8 of a 21-day cycle. Patients received the study treatment unless disease progression, unacceptable toxicity, or a request to discontinue from the patient and/or investigator eventuated.
Results
Between December 2012 and September 2015, 32 patients with metastatic breast cancer were enrolled at 10 participating clinical institutions in Japan, and toxicity and response rates were evaluated. The overall response rate was 43.8% (95% confidence interval 26.5–61.0). The clinical benefit and tumor control rates were 56.3% (95% CI 39.0–73.5) and 78.1% (95% CI 63.8–92.5), respectively. Median progression-free survival was 8.3 months (95% CI 7.1–9.4). A subgroup analysis did not identify any factors affecting the efficacy of eribulin. The most common adverse events were neutropenia (71.9%), alopecia (68.7%), and peripheral neuropathy (46.9%). As a first- or second-line therapy, eribulin showed sufficient efficacy for metastatic breast cancer compared with taxane and capecitabine treatment in previous clinical trials. The safety profile of eribulin was acceptable.
Conclusions
Eribulin may be another option for first-line chemotherapeutic regimens for metastatic breast cancer.
Trial registrations
This trial was retrospectively registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (ID number: UMIN000010334).
Date of trial registration: April 1st, 2013.
References:
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol. 2013;24(9):2206–23.View ArticlePubMedPubMed CentralGoogle Scholar
- Colozza M, de Azambuja E, Personeni N, Lebrun F, Piccart MJ, Cardoso F. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007;12(3):253–70.View ArticlePubMedGoogle Scholar
- Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086–95.View ArticlePubMedGoogle Scholar
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–23.View ArticlePubMedGoogle Scholar
- Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE, Cortes J. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33(6):594–601.View ArticlePubMedPubMed CentralGoogle Scholar
- Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, Awada A. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148(3):553–61.View ArticlePubMedPubMed CentralGoogle Scholar
- Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, Gralow J, Hortobagyi GN, Moy B, Yee D et al: Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014, 32(29):3307–3329.Google Scholar
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76.View ArticlePubMedGoogle Scholar
- Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS, Nowak AK, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29(34):4498–504.View ArticlePubMedGoogle Scholar
- Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, Uesugi M, Agoulnik S, Taylor N, Funahashi Y, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110(6):1497–505.View ArticlePubMedPubMed CentralGoogle Scholar
- Agoulnik SI, Kawano S, Taylor N, Oestreicher J, Matsui J, Chow J, Oda Y, Funahashi Y. Eribulin mesylate exerts specific gene expression changes in pericytes and shortens pericyte-driven capillary network in vitro. Vasc Cell. 2014;6(1):3.View ArticlePubMedPubMed CentralGoogle Scholar
- Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, Kuji I, Osaki A. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer. 2016;114(11):1212–8.View ArticlePubMedPubMed CentralGoogle Scholar
- McIntyre K, O'Shaughnessy J, Schwartzberg L, Gluck S, Berrak E, Song JX, Cox D, Vahdat LT. Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat. 2014;146(2):321–8.View ArticlePubMedPubMed CentralGoogle Scholar
- Takashima T, Tokunaga S, Tei S, Nishimura S, Kawajiri H, Kashiwagi S, Yamagata S, Noda S, Nishimori T, Mizuyama Y, et al. A phase II, multicenter, single-arm trial of eribulin as first-line chemotherapy for HER2-negative locally advanced or metastatic breast cancer. Springerplus. 2016;5:164.View ArticlePubMedPubMed CentralGoogle Scholar
Copyright
Gerelateerde artikelen
- Studiepublicaties van niet-toxische middelen en behandelingen uit literatuurlijst van arts-bioloog drs. Engelbert Valstar, gerelateerd aan borstkanker copy 1
- ESMO 2023: aanbevolen abstracten voor borstkanker door oncologen en borstkankerspecialisten
- ASCO 2024: aanbevolen abstracten gerelateerd aan borstkanker door vooraanstaande oncologen wereldwijd
- 177Lu-FAP-2286 geeft uitstekende resultaten bij zwaar voorbehandelde patienten met vergevorderde uitgezaaide hormoongevoelige borstkanker waar verder geen behandelingsopties meer voorhanden waren
- Algemeen: Borstkanker korte algemene informatie
- Aprepitant, een antibraak middel bij chemotherapie, blijkt betere ziektevrije overleving op afstand en borstkankerspecifieke overleving te geven bij borstkankerpatienten met borstkanker in vroeg stadium
- Arimidex - anastrozole informatie en studie overzicht
- Arimidex: 2 jaar aromataseremmers na hormoontherapie bij borstkanker geeft dezelfde overall overleving als 5 jaar. Veel borstkankerpatienten worden overbehandeld. copy 1
- Artemisinin - Armesia: informatie over Artemisinin, als medicijn succesvol bij malaria maar ook als aanvullend middel bij vormen van kanker waaronder borstkanker bij elkaar gezet.
- Avastin in combinatie met verschillende vormen van chemo bij borstkanker geeft langere ziektevrije tijd maar geen verschil in mediane overlevingstijd noch in overall overlevingen. FDA trekt goedkeuring definitief in
- Bestraling bij borstkanker: een overzicht van belangrijke artikelen en recente studies.
- Bisfosfonaten geven een betere algehele overleving en borstkankerspecifieke overleving bij oudere vrouwen (+65 jaar). Denosumab geeft geen extra verbetering van overall overleving.
- Boeken gerelateerd aan borstkanker
- Buparsilib een PI3K remmer naast fulvestrant geeft 30 tot 50 procent langere ziekteprogressievrije tijd maar wel meer bijwerkingen bij gevorderde borstkanker copy 1
- Chemo bij borstkanker: hier een overzicht van belangrijke artikelen en recente studies bij elkaar gezet.
- Chinese kruiden verbeteren bijna altijd de effecten van chemo en verminderen eigenlijk altijd de bijwerkingen ervan. Klik hier voor artikelen over TCM - Traditionele kruiden.
- ctDNA - circulerend DNA in bloed meten geeft betere prognose op resultaten en effectiviteit van behandelingen bij borstkanker type HER2 positief dan complete remissies
- Datopotamab deruxtecan geeft bij patiënten met inoperabele of uitgezaaide lokale triple-negatieve borstkanker voor wie immuuntherapie geen optie was uitstekende resultaten in vergelijking met chemotherapie
- Dendritische celtherapie. Ook geschikt voor borstkanker.
- Denosumab injecties geven ook bij borstkanker uitstekende resultaten tegen botuitzaaiingen
- Diagnose en oorzaken van borstkanker: een overzicht van artikelen en recente ontwikkelingen.
- Enzalutamide - Xtandi geeft spectaculaire resultaten bij gevorderde triple negatieve borstkanker (stadium 4) met ook een positieve expressie van de hormoonreceptor. copy 1
- Eierstokken weghalen naast standaard behandelingen van chemo, borstoperatie, bestraling, hormoontherapie geeft 40 procent betere overleving voor vrouwen met BRCA2-gemuteerde borstkanker stadium I – III
- Eribuline mesylaat is net zo effectief of beter dan chemo als eerste- of tweedelijnsbehandeling voor lokaal gevorderde of uitgezaaide borstkanker
- Ervaringsverhalen Borstkanker: Vrouw van 29 jaar met triple negatieve borstkanker stadium IV komt in complete remissie door aanvullend op chemo persoonlijke voedingsadviezen (kytogeen dieet), hyperthermie en hyperbare zuurstoftherapie copy 1 copy 1
- Everolimus (Afinitor) plus hydroxychloroquine doodt slapende tumorcellen bij borstkankerpatienten en geeft zeer goede ziektevrije overleving en overall overlevingsresultaten van 85 tot 100 procent blijkt uit de fase II studie CLEVER.
- Exemestane - Aromasin als behandelings medicijn bij vrouwen met borstkanker in de leeftijd na de overgang..
- Gebapentin een niet hormonaal medicijn effectief tegen opvliegers bij vrouwen met borstkanker. die een hormoon behandeling volgen.
- Genentest: Oncotype DX - Genetest voorspelt kansen op recidief bij hormoongevoelige borstkanker met geen uitzaaiingen door behandeling met chemokuren. Aldus twee tienjarige studies gepubliceerd in de NEJM - New England Journal of Medicin
- Herceptin - Trastuzumab bij borstkanker: een overzicht van recente ontwikkelingen en belangrijke artikelen en studies
- Hormoontherapie plus CDK 4/6 remmers geeft beste resultaten op progressievrije ziekte en overall overleving voor patienten met borstkanker met hormoongevoelige uitgezaaide borstkanker en HER-2 neg. in vergelijking met alleen hormoontherapie of chemo.
- Hyperthermie geeft significant betere resultaten bij verschillende kankersoorten en vooral bij borstkankerpatienten die eerder bestraald zijn.
- Immuuntherapie bij borstkanker, een overzicht
- Iressa - gefinitib toegevoegd aan arimidex - anastrozol geeft langere ziektevrije tijd en mediane overleving voor patienten met hormoongevoelige borstkanker. Artikel geplaatst 1 april 2010
- Lapatinib - Tykerb naast herceptin - trastuzumab geeft significant langere ziektevrije tijd en betere overall overleving dan alleen herceptin bij patienten met borstkanker met positieve Her2-Neu expressie, maar faalt bij beginnende borstkanker
- Lipidenverlagende medicijnen (LLM) (statines) hebben gunstige invloed op overall overleving van kankerpatienten met borstkanker, darmkanker en melanomen. copy 1
- LITT behandeling - Laser-induced Interstitial Thermotherapy - bij borstkanker geeft ook significant betere en langere overlevingstijd bij levertumoren vanuit borstkanker
- Lymfoedeem ontstaan direct na een operatie van okselklieren, na bestraling van de oksel ontstaat lymfoedeem vaak enkele jaren later..
- Mammaprint - een genentest die kan voorspellen welke behandeling een borstkankerpatient nodig heeft.
- Mammastatin: Is mammastatin de nieuwste doorbraak bij borstkanker? Een bepaald eiwit zou effect van visolie bij borstkanker zodanig stimuleren dat ontwikkeling van borstkankercellen wordt gestopt en zelfs vernietigd.
- NABON - Nationaal Borstkanker Overleg Nederland stelt: overgrote deel van Nederlandse ziekenhuizen - 85% - voldoet niet aan de norm tot een adekwate behandeling en zorg voor borstkankerpatiënten binnen een daarvoor gestelde termijn.
- Newcastle virus: Dierproeven met Newcastlevirus bij borstkankerpatiënten HER2-Neu bepaald lijken hoopvol.
- Oorzaak borstkanker: Gen ontdekt dat mogelijk rol speelt bij ontstaan van borstkanker
- Operatie: aantal artikelen over operatie technieken en gevolgen van operatieve ingrepen bij borstkanker bij elkaar gezet
- Opvliegers tijdens ziekte blijken betere prognose op genezing van borstkanker te geven en geven bijna 50 procent minder kans op een recidief. 12,9 procent tegenover 21 procent..
- Osteoporose - botontkalking bij borstkanker: Hormoontherapie anders dan met tamoxifen geeft groter risico op botontkalking bij borstkankerpatiënten, ook als ze borstkanker overleven.
- Overlevenden van borstkanker hebben sterk verhoogd risico - 25 procent - op krijgen van andere vormen van kanker dan borstkanker in vergelijking met de doorsnee bevolking
- PARP remmers als monotherapie voor BRCA-1 en BRCA 2 pos. plus HER2-neg. uitgezaaide borstkanker geeft wel langere progressievrije ziekte maar geen verschil in overall overleving in vergelijking met chemotherapie
- Patientenervaringen: Borstkanker - Casestudies
- PDT - Photo Dynamische Therapie kan ook bij borstkanker:een optie zijn, vooral die eerder al geopereerd en bestraald zijn.
- Prof. dr. Pinedo over aanpak van borstkanker en andere solide tumoren. Lezing uit 2001. Nieuwe gegevens over GM-GSF studies toegevoegd.
- Pyrotinib na falen van lapatinib (Tykerb) kan toch nog behandelingsoptie zijn voor patiënten met HER2-positieve uitgezaaide borstkanker, zelfs bij hersenmetastases
- Radium-223, bij prostaatkanker effectief, lijkt ook voor in botten uitgezaaide borstkanker een uitstekend medicijn
- RFA - Radio Frequency Ablation bij borstkanker: enkele artikelen bij elkaar gezet.
- Sacituzumab tirumotecan (Trodelvy) geeft alsnog uitstekende resultaten bij patienten met voorbehandelde triple negatieve borstkanker waar hormoontherapie resistentie optrad in vergelijking met chemotherapie
- Sacituzumab govitecan (Trodelvy) een anti-lichaam medicijn geeft zeer goede resultaten bij borstkankerpatiënten met voorbehandelde uitgezaaide triple-negatieve borstkanker met TROP-2 mutatie in vergelijking met chemotherapie
- Schildwachtkliermethode bij borstkanker
- Siliconen en risico op borstkanker geven siliconen nu wel of niet een groter risico op borstkanker? Hier een uitgebreide analyse en historisch overzicht.met veel studies die ernaar gedaan zijn
- Sonia studie: Borstkanker wordt vaak overbehandeld. Erasmus MC doet daar wat aan via de Sonia studie. copy 1
- Stamcelbehandeling (autologous stem cell rescue) plus chemokuren bij borstkanker geven superieure overleving t.o.v. een enkele stamcelbehandeling.
- TACE - Transarteriële chemo-embolisatie - bij borstkankerpatiënten voor hun leveruitzaaiïngen verlengt het leven van deze patiënten significant.
- TACP - Trans Arteriële Chemo Perfusie geeft bij gevorderde uitgezaaide borstkanker nog betere resultaten dan bij darmkanker copy 1
- Tetracycline bij verschillende vormen van kanker als botversterker en bestrijder van kanker in de botten lijkt een interessant middel.
- Ultra Sound - opereren via geluid en verhitting - wordt ingezet bij beginnende borstkanker in het UMC - Utrecht copy 1
- Vermoeidheid bij overlevenden van borstkanker: 32% van de vrouwen die borstkanker overleven heeft tot minimaal tien jaar na de diagnose last van ernstige vermoeidheidsverschijnselen blijkt uit tienjarige grote studie
- Zelfonderzoek naar borstkanker leidt niet tot langere overlevings duur maar wel tot grotere medicalisering.
- Zometa - Zoledronic acid informatie bij elkaar gezet.
- Reguliere behandelingen, medicijnen en middelen bij borstkanker: een overzicht



Plaats een reactie ...
Reageer op "Eribuline mesylaat is net zo effectief of beter dan chemo als eerste- of tweedelijnsbehandeling voor lokaal gevorderde of uitgezaaide borstkanker"