Aan dit artikel is enkele uren gewerkt. Beoordelen, opzoeken relevante informatie, uitleggen, vertalen en plaatsen op de website. Mocht u ons willen ondersteunen om kanker-actueel online te houden zodat we meer van dit soort artikelen kunnen bijven publiceren dan kunt u ons helpen via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel Terneuzen.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven dus voor allemaal een win win situatie:
26 september 2017:J Clin Invest. 2016 Jun 1; 126(6): 2334–2340.
De laatste jaren worden veel studies gepubliceerd waarin kankerpatiënten met veel verschillende vormen van kanker alsnog goed reageren op immuuntherapie met een anti-PD medicijn, zoals de inmiddels voor sommige vormen van kanker goedgekeurde pembrolizumab, nivolumab, atezolizumab, avelumab enz.. En wie zoekt in clinical trials ziet dat er nog tientallen andere vergelijkbare medicijnen in onderzoek zijn, allemaal meestal in naam eindigend op......mab.
Deze vormen van immuuntherapie met zogeheten antilichamen zijn gericht op het zogeheten immune checkpoint receptor programmed cell death protein 1 (PD-1). Deze receptor (P1-ligand) zorgt ervoor dat er geen apoptose - zelfdoding plaatsvindt van beschadigde kankercellen of als cellen hun werk hebben verricht, die daardoor uit kunnen groeien tot tumoren want ze blijven maar delen. De immuuntherapie met anti-PD medicijnen schakelt die receptoren (PD-1 ligand) als het ware weer in zodat het immuunsysteem de beschadigde of uitgewerkte cellen kan vernietigen / verwijderen.
Uit studies blijkt dat als deze vorm van immuuntherapie aanslaat er langdurige complete remissies kunnen ontstaan met vaak ook echt genezing. Echter een anti-PD-1 therapie heeft niet bij alle patiënten een goed en volledig effect en kan soms ook tot nadelige bijwerkingen leiden. De factoren die bepalen waarom patiënten gevoelig of juist resistent zijn voor een anti-PD aanpak, worden nog niet helemaal begrepen. Maar wordt wel steeds verder via genomische profilering - receptoren en DNA mutatie onderzoek - onderzocht en de onderzoekers komen steeds verder daarmee.
Bv. in deze studie, gepresenteerd op ESMO 2016: Mutations in the POLE proofreading domain identifiy a subset of colorectal cancers that have enhanced immunogenicity blijkt dat ook andere mutaties een prognose kunnen geven op aanslaan of niet. En vooral dat deze darmkankerpatiënten langere ziektevrije tijd en overall overleving hadden. Onder grafiek andere studie bij darmkanker en twee casestudies van vrouwen met darmkanker en buikvliestumoren met bepaalde mutaties.
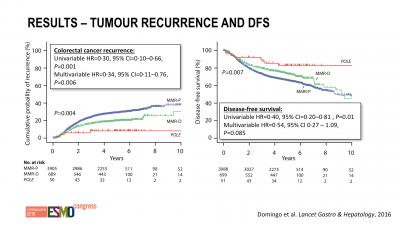
Interessant is bv. dat een andere mutatie, de POLE mutatie genoemd (DNA polymerase epsilon), een belangrijk inzicht kan geven in het eventueel wel of niet reageren op een anti-PD behandeling.
Zo werd onlangs deze studie gepresenteerd: Immunotherapy for Colorectal Cancer waarin wordt beschreven welke mutaties een rol spelen bij immuuntherapie met anti-PD medicijnen. Op dit moment wordt de MSI-H bij darmkanker gezien als een voorspeller voor aanslaan van immuuntherapie met anti-PD medicijnen (checkpointremmers) maar er zijn er dus meer:
Uit dit studierapport: The majority of colorectal cancers demonstrate activation of the wnt/B-catenin pathway, in part due to inactivation of the tumor suppressor gene, APC. Relevant to therapeutic targeting, in metastatic disease RAS (KRAS or NRAS), mutations are seen in over 50% of patients, with BRAF mutations seen in 5–10% [3,4]. Additional emerging targets include HER-2 amplifications, seen in 2–5% of all colorectal cancers [5]. ............
A subset of colorectal cancers possesses markedly elevated mutational rates. Predominantly, these tumors are characterized by dysfunction of the mismatch repair genes (microsatellite high or MSI-H). MSI-H tumors make up a minority of colorectal cancers, with decreasing frequency in more advanced stage disease. The prevalence of MSI-H in stage II, III and IV colorectal cancers stands at 22%, 12%, and 3%, respectively [7,8]. A small fraction of hyper-mutated tumors possesses polymerase mutations, specifically within the catalytic domain of DNA polymerase epsilon (POLE) or delta (POLD1). These hypermutated tumors are of great relevance in our current understanding of colorectal cancer subtyping and the role of immunotherapy.
Hier een overzicht van enkele belangrijke studies van afgelopen jaren bij darmkanker met bepaalde mutaties van immuuntherapie met anti-PD medicijnen. (Tekst gaat verder onder grafiek)
| Drug(s) | Target | Population | Patients | Response Rate | Identifier |
|---|---|---|---|---|---|
| Trials for MSI-H CRC | |||||
| Pembrolizumab | PD-1 | Refractory MSI-H CRC | 25 | 57% | Le et al. [30] |
| Nivolumab Nivolumab + Ipilimumab | PD-1 | Refractory MSI-H CRC | 47 | 26% | NCT02060188 [31] |
| PD-1 + CTLA-4 | Refractory MSI-H CRC | 30 | 33% | ||
| Trials for MSS CRC | |||||
| Pembrolizumab | PD-1 | Refractory MSS CRC | 28 | 0% | Le et al. [30] |
| Nivolumab + Ipilimumab | PD-1 + CTLA-4 | Refractory MSS CRC | 20 | 5% | NCT02060188 [31] |
| Trials of Various CRC Sub-Types | |||||
| Tremelimumab | CTLA-4 | Refractory CRC | 49 | 2% | Chung et al. [28] |
| Nivolumab | PD-1 | Refractory CRC | 19 | 0% | Topalian et al. [32] |
| BMS-936559 | PD-L1 | Refractory CRC | 18 | 0% | Brahmer et al. [33] |
| Atezolizumab + Bevacizumab | PD-L1 | Refractory CRC | 14 | 7% | NCT01633970 [34] |
| Atezolizumab + FOLFOX/bev | Metastatic CRC (70% first line) |
30 | 40% (total) 48% (first-line) |
||
| Atezolizumab + Cobimetinib | PD-L1 MEK | Refractory CRC (30% MSS, 70% unknown) | 23 | 17% (3 MSS, 1 unknown) | NCT01988896 [35] |
Op ESMO 2017 werd een studie gepresenteerd waarin onderzoekers een vrouw met een geschiedenis van kanker met buikvliestumoren analyseerden. Zij reageerde uiteindelijk na vele chemokuren heel goed op immuuntherapie met het anti-PD-1 medicijn pembrolizumab. Een analyse van de Cancer Genome Atlas (TCGA) liet zien dat de aanwezigheid van een POLE mutatie gerelateerd bleek aan een verhoogde expressie van verschillende checkpoint controle genen. Samen tonen deze gegevens aan dat tumoren die POLE-mutaties bevatten, goede kandidaten zijn voor immuuntherapie met anti-PD medicijnen.
Het volledige studierapport van deze vrouw, een casestudie: Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer is gratis in te zien. Onderaan dit artikel het abstract plus referentielijst.
Een ander voorbeeld van een vrouw met gevorderde darmkanker met een POLE mutatie die uitstekend reageerde is dit studierapport: Exceptional Chemotherapy Response in Metastatic Colorectal Cancer Associated With Hyper-Indel–Hypermutated Cancer Genome and Comutation of POLD1 and MLH1 dat ook gratis is in te zien. Abstract eveneens onderaan dit artikel.
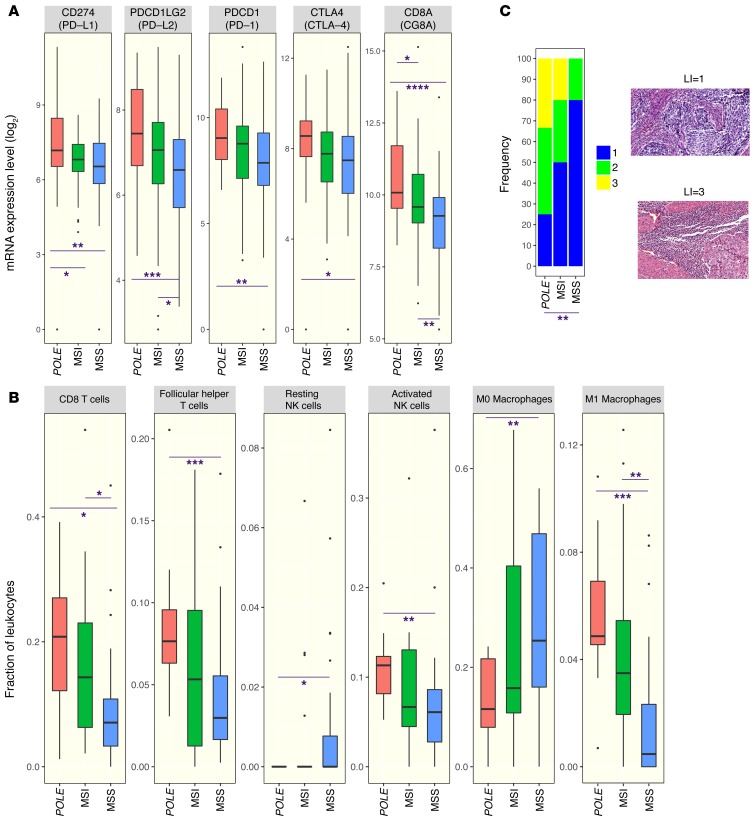
Hier de abstracten van eerder genoemde studies met referentielijsten enz.
Here, we review the contemporary understanding of the immune and molecular landscape in colorectal cancer and discuss ongoing clinical trials evaluating novel combination regimens based on immune checkpoint inhibitors.
Immunotherapy for Colorectal Cancer
Abstract
The recent success of anti-PD1 drugs in metastatic colorectal cancer patients with mismatch repair deficiency generated overwhelming enthusiasm for immunotherapy in the disease. However, patients with mismatch repair deficient colorectal cancer represent only a small subset of the metastatic population. Current research focuses on advancing immunotherapy to earlier stages of the disease including adjuvant and first-line metastatic settings, and on inducing sensitivity to immune checkpoint inhibitor therapy through a combinatorial approach. Here, we review the contemporary understanding of the immune and molecular landscape in colorectal cancer and discuss ongoing clinical trials evaluating novel combination regimens based on immune checkpoint inhibitors.
5. Conclusions
The American Society of Clinical Oncology (ASCO) declared Immunotherapy to be the 2016 Clinical Cancer Advance of the Year. In 2017, the advance of the year has already been announced to be Immunotherapy 2.0. Despite years of frustration, we are beginning to see some success through the use of the immunotherapy approach in colorectal cancer, namely PD-1 inhibition in MSI-H cancers. However, the successful targeting of MSS cancers and non-hypermutated tumors appears to be not too far off on the horizon. MEK and PD-L1 combinations are being rigorously tested, multiple agents and combinations are in development and multiple companies have shifted their focus and investments toward immunotherapeutics. Neglected in this review, but also of note, a case of remarkable success has been witnessed utilizing adoptive cell therapy via tumor infiltrating lymphocytes (TILs) in colorectal cancer [52]. Thus, cancer immunotherapy strategies appear to be moving full speed ahead. Despite the knowledge that many further failures lie in our paths, reason for great optimism remains.
Conflicts of Interest
Patrick M. Boland has received research funding from Merck. Wen Wee Ma has no conflicts of interest to declare.
References
POLE proofreading domain mutations identify a subset of immunogenic colorectal cancers with excellent prognosis. This association underscores the importance of rare biomarkers in precision cancer medicine, but also raises important questions about how to identify and implement them in practice.
Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study
Summary
Background
Precision cancer medicine depends on defining distinct tumour subgroups using biomarkers that may occur at very modest frequencies. One such subgroup comprises patients with exceptionally mutated (ultramutated) cancers caused by mutations that impair DNA polymerase epsilon (POLE) proofreading.
Methods
We examined the association of POLE proofreading domain mutation with clinicopathological variables and immune response in colorectal cancers from clinical trials (VICTOR, QUASAR2, and PETACC-3) and colorectal cancer cohorts (Leiden University Medical Centre 1 and 2, Oslo 1 and 2, Bern, AMC-AJCC-II, and Epicolon-1). We subsequently investigated its association with prognosis in stage II/III colorectal cancer by Cox regression of pooled individual patient data from more than 4500 cases from these studies.
Findings
Pathogenic somatic POLE mutations were detected in 66 (1·0%) of 6517 colorectal cancers, and were mutually exclusive with mismatch repair deficiency (MMR-D) in the 6277 cases for whom both markers were determined (none of 66 vs 833 [13·4%] of 6211; p<0·0001). Compared with cases with wild-type POLE, cases with POLE mutations were younger at diagnosis (median 54·5 years vs 67·2 years; p<0·0001), were more frequently male (50 [75·8%] of 66 vs 3577 [55·5%] of 6445; p=0·0010), more frequently had right-sided tumour location (44 [68·8%] of 64 vs 2463 [39·8%] of 6193; p<0·0001), and were diagnosed at an earlier disease stage (p=0·006, χ2 test for trend). Compared with mismatch repair proficient (MMR-P) POLE wild-type tumours, POLE-mutant colorectal cancers displayed increased CD8+ lymphocyte infiltration and expression of cytotoxic T-cell markers and effector cytokines, similar in extent to that observed in immunogenic MMR-D cancers. Both POLE mutation and MMR-D were associated with significantly reduced risk of recurrence compared with MMR-P colorectal cancers in multivariable analysis (HR 0·34 [95% CI 0·11–0·76]; p=0·0060 and 0·72 [0·60–0·87]; p=0·00035), although the difference between the groups was not significant.
Interpretation
POLE proofreading domain mutations identify a subset of immunogenic colorectal cancers with excellent prognosis. This association underscores the importance of rare biomarkers in precision cancer medicine, but also raises important questions about how to identify and implement them in practice.
Funding
Cancer Research UK, Academy of Medical Sciences, Health Foundation, EU, ERC, NIHR, Wellcome Trust, Dutch Cancer Society, Dutch Digestive Foundation.
Analysis of The Cancer Genome Atlas (TCGA) revealed that the presence of POLE mutation associates with high mutational burden and elevated expression of several immune checkpoint genes. Together, these data suggest that cancers harboring POLE mutations are good candidates for immune checkpoint inhibitor therapy.
Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer
Abstract
Antibodies that target the immune checkpoint receptor programmed cell death protein 1 (PD-1) have resulted in prolonged and beneficial responses toward a variety of human cancers. However, anti–PD-1 therapy in some patients provides no benefit and/or results in adverse side effects. The factors that determine whether patients will be drug sensitive or resistant are not fully understood; therefore, genomic assessment of exceptional responders can provide important insight into patient response. Here, we identified a patient with endometrial cancer who had an exceptional response to the anti–PD-1 antibody pembrolizumab. Clinical grade targeted genomic profiling of a pretreatment tumor sample from this individual identified a mutation in DNA polymerase epsilon (POLE) that associated with an ultramutator phenotype. Analysis of The Cancer Genome Atlas (TCGA) revealed that the presence of POLE mutation associates with high mutational burden and elevated expression of several immune checkpoint genes. Together, these data suggest that cancers harboring POLE mutations are good candidates for immune checkpoint inhibitor therapy.
Reference information:J Clin Invest. 2016;126(6):2334–2340. doi:10.1172/JCI84940.
References
Gerelateerde artikelen
- 6 nieuwe doorbraken in de strijd tegen kanker worden gepresenteerd door het World Economic Forum met bijbehorende video
- 90 procent van mensen met uitgezaaide kanker heeft meerdere DNA afwijkingen. Slechts 5 procent kreeg ook optimale behandeling daarvoor.
- Antibiotica binnen een maand vooraf aan immuuntherapie met anti-PD medicijnen geeft veel slechtere resultaten op overall overleving dan zonder antibiotica bij verschillende vormen van primaire kanker.
- Anti-PD medicijnen zoals nivolumab, Pembrolizumab en atezolizumab gegeven als immuuntherapie geven zeer goede resultaten bij verschillende vormen van kanker met solide tumoren, zelfs zonder Ligand-1 receptorstatus copy 1
- Bacterien in uitzaaiingen van kankerpatienten zijn door Nederlandse onderzoekers in beeld gebracht en in een gedetailleerde catalogus opgeslagen
- Behandelen van kanker verschuift steeds meer van chemotherapie naar biologische behandelingen, gerichte therapie waaronder immuuntherapie met gemoduleerde virussen die de minste bijwerkingen geven
- Biomarkers zoals PD-L1, CD163+ en NRAS mutaties en gegevens zoals uitzaaiingen later ontstaan bepalen kans van effectiviteit van immuuntherapie met anti PD medicijnen bij melanomen
- Bloedtesten, een overzicht van recent gepubliceerde resultaten van verschillende bloedtesten
- CRISPR-Cas kan veranderingen in bacterien bewerkstelligen en via bewerkte bacterien verspreiden om zo resistentie van antibiotica te veranderen in opnieuw werkende antibiotica.
- CHRISPR-CAS9 infuus blijkt genezende behandeling voor erfelijke aandoening angio-oedeem, aldus tussenresultaten van internationale studie met Nederlandse deelname.
- CLEVER studie: Waarom kanker jaren later kan terugkeren - en hoe dit te voorkomen. CLEVER studie bij borstkankerpatienten bewijst dat een recidief is te voorkomen door tumorcellen in beenmerg te behandelen.
- De biologische processen waarom en hoe kankercellen uitzaaien wordt beter begrepen, tumorcellen vroeger ontdekt en lijkt ook steeds beter te behandelen
- De huidige staat van moleculair testen in het behandelen van kankerpatienten met solide tumoren. Een uitstekend overzichtsartikel met de nieuwste ontwikkelingen over RNA, DNA en eiwitten anno 2019
- Diagnosetest PERCEPTION via AI - Kunstmatige Intelligentie ontwikkeld en met hulp van single-cell RNA-sequencing voorspelt nauwkeurig of een specifiek medicijn van de kankerpatient zal aanslaan of resistent zal zijn.
- DRUP studie geeft bij 37 procent van de patienten alsnog een therapeutisch effect met 6 procent CR en 14 procent PR en 17 procent stabiele ziekte
- EMA: Veel nieuwe kankermedicijnen in de EU hebben geen bewezen toegevoegde waarde blijkt uit Nederlandse studie naar goedgekeurde kankermedicijnen door het Europees Geneesmiddelenbureau (EMA).
- Erfelijkheid van kanker hangt vaak af van specifieke afwijkende genencombinaties in DNA onderzoek en eiwitexpressie blijkt uit groot Whole Exome Sequencing onderzoek via de Biobank van de UK.
- ESMO - European Society for Medical Oncology heeft een gids uitgegeven voor patienten over hoe personalised medicine werkt en stand van zaken
- FDA ondersteunt onderzoek naar personalised medicine op basis van mutaties ongeacht in welk lichaamsdeel de kanker zich het eerst openbaart.
- Genetisch onderzoek via Germline testen (kiembaan testen) werd in periode 2013 tot 2019 in Georgie en Californie bij slechts 7 procent gedaan onder 1 369 602 patienten met twee jaar kanker.
- Genetische mutatie ontdekt die kans van slagen van immuuntherapie bij alle vormen van kanker naar 100 procent zou kunnen brengen
- Geneesmiddel (ARS1620) verandert kankergen (KRAS mutatie) dat kwaadaardige tumoren beschermt tegen immuunsysteem in een doelwit voor immuunsysteem en helpt immuuntherapie kankercellen te elimineren
- Gentherapie zoals Chrispr-cas en base-editors zijn zeer succesvol bij erfelijke ziekten waaronder ook vormen van kanker zoals sikkelcelziekte
- Gerichte behandelingen met Aurora kinaseremmers geven soms uitstekende resultaten bij veel vormen van kanker. Een reviewstudie
- Immuunafwijkingen bij kankerpatienten gerelateerd aan infecties voortijdig ontdekken zouden in behandelingen van kanker sterven aan kanker met 25 procent of meer kunnen voorkomen
- Immuuntherapie met HER2-gerichte CT-0508 (CAR-Macrofaag therapie) geeft bij solide tumoren van verschillende vormen van kanker met HER2 positieve expressie hoopvolle resultaten
- Immuuntherapie met pembrolizumab bij patiënten met verschillende vormen van uitgezaaide kanker met hoge microsatellietinstabiliteit (MSI-H) en DNA-mismatch-reparatie-deficiënte (dMMR) geeft uitstekende en duurzame resultaten op overall overleving
- Immuuntherapie met nivolumab zorgt voor duurzame en sterk verbeterde overall overleving bij verschillende vormen van kanker, melanomen, longkanker en nierkanker copy 1
- Interleukin-15 speelt een cruciale rol wanneer gegeven samen met immuuntherapie voor verschillende vormen van kanker met solide tumoren en biedt veelbelovende mogelijkheden voor verbeterde behandelingen
- Internationale groep van 180 wetenschappers stelt rapport op hoe en met welke niet-toxische middelen - voedingsstoffen de effectiviteit te verbeteren, recidieven te voorkomen en de bijwerkingen te verminderen van personalised medicine
- Irina Kareva gebruikt wiskundige modellen die de dynamiek van kanker beschrijven, met het doel nieuwe geneesmiddelen te ontwikkelen die gericht zijn op tumoren.
- Kanker-actueel kan en wil helpen - begeleiden bij aanvragen van een volledig biomoleculair receptorenonderzoek en genenonderzoek
- Erfelijkheid: Kanker is vaak domme pech stelt prof. dr. Nicoline Hoogerbrugge van het Radboudumc en de Radboud Universiteit in een interview in de Stentor en Algemeen Dagblad
- Kankermedicijnen geven in de klinische praktijk veel minder effect dan uit de studies van farmaceutische bedrijven is aangetoond. Maar zijn wel ontzettend duur.
- Kankerremmende eiwitten kunnen bij mutatie die gen uitschakelt veranderen van kankerremmend in stimulerend, ontdekten Nederlandse onderzoekers
- Larotrectinib geeft bijzonder goede resultaten (76 procent respons met 12 procent complete remissies) bij alle vormen van solide tumoren met een positieve TRK Fusion mutatie
- Larotrectinib: Met de goedkeuring van Larotrectinib op basis van 1 specifieke afwijking en niet op basis van primaire tumor zorgt de FDA voor een doorbraak in het behandelen van kanker
- Lenvatinib Plus Pembrolizumab bij patiënten met inoperabele gevorderde nierkanker, buikvlieskanker, melanomen en andere gevorderde kanker met solide tumoren geeft uitstekende resultaten met meer dan de helft remissies van 50 procent of meer copy 1
- Medicijnen voorschrijven op basis van DNA profiel van de patient voorkomt 30 procent minder bijwerkingen blijkt uit internationale studie onder leiding van LUMC Leiden
- Moleculaire schakelaar verandert kankercellen in weer normale cellen en zou genezende aanpak van kanker kunnen betekenen
- MSC-1 een medicijn dat de groei van de kankerstamcellen afremt door LIF blokkade en immuunsysteem activeert laat spectaculair goede resultaten zien in fase I studie.
- Mytomorrows breidt aanbod aan experimentele medicijnen voor kankerpatienten uit met 11 nieuwe nog niet geregistreerde medicijnen en stelt deze beschikbaar voor uitbehandelde kankerpatienten
- Nederland betaalt veel meer voor kankermedicijnen, soms tot 50 procent of meer, dan andere landen blijkt uit vergelijkend onderzoek tussen 18 landen copy 1
- NCI-MATCH-studie toont aan dat een biomoleculaire analyse - DNA en receptorenonderzoek - belangrijk is in hoe een kankerpatient te behandelen.
- Nieuw medicijn - PD-0332991 - stopt groei hersentumoren Glioblastoom in dierproeven. Zodra gestopt werd met dit medicijn gingen de tumoren weer groeien. Fase I studie bij 33 patienten met nierkanker en lymfklierkanker bevestigt veiligheid van dit middel
- Nieuwe, dure kankermedicijnen zijn voortaan sneller beschikbaar door het Drug Access Protocol (DAP) dat is ontwikkeld door oncologen, verzekeraars en Zorginstituut Nederland
- Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie copy 1
- Overzicht van studies met medicijnen en behandelingen om tumoren met KRAS mutaties aan te pakken. Vooral combinatiebehandelingen zijn veelbelovend.
- PI3K/AKT/mTOR pathway speelt cruciale rol in apoptose proces, DNA herstel, metabolisme in de cel en angiogenese.
- Pembrolizumab - Keytruda geeft bij solide tumoren van verschillende oorsprong 21 procent complete remissies en 53 procent gedeeltelijke remissies.
- Personalised medicine door receptorenonderzoek geeft veel betere resultaten in fase 1 studies dan experimenteel onderzoek zonder receptorenonderzoek
- Prof. Bernards over de doorbraak bij darmkanker met Kras mutatie en bij melanomen met BRAF mutatie in DWDD van donderdag 27 maart 2014
- POLE mutatie: veel kankerpatienten met erfelijke vormen van kanker hebben naast een P1-ligand een POLE mutatie en reageren goed op immuuntherapie met anti-PD medicijnen - checkpointremmers als pembrolizumab en nivolumab
- Radiotherapeutisch stimulerend middel NBTXR3 geeft in combinatie met anti-PD-1 medicijnen alsnog uitstekende resultaten bij patiënten die ziekteprogressie lieten zien ongeacht eerdere behandeling met anti-PD-1 medicijnen
- Rezatapopt, een p53-reactivator gericht op de p53 Y220C-mutatie, geeft in veiligheidstudie hoopvolle resultaten bij zwaarvoorbehandelde kankerpatienten met solide tumoren
- Rozlytrek (entrectinib), een tyrosine kinase remmer, goedgekeurd door FDA als medicijn voor solide tumoren met NTRK (neurotrophic tyrosine receptor kinase) gene fusion. Dit is 3e goedgekeurde medicijn op basis van mutatie.
- Stamceltherapie succesvol toegepast bij vrouw met diabetes type 1. Zij hoeft nu al een jaar geen insuline meer te spuiten.
- Tweede primaire vorm van kanker bij een kankerpatient wordt steeds vaker bekend bij de diagnose (2 tot 17 procent) door betere diagnose technieken en verfijnder biomoleculair onderzoek
- Tumorindeling mede aan de hand van biomarkers - biomoleculaire profielen is nodig en zal behandelingen sterk veranderen voor veel kankerpatiënten. Van 10 procent nu tot 50 procent straks. Aldus grote studie van het TOGA
- Vaccin tegen KRAS positief gemuteerde vormen van kanker - darmkankers en longkanker o.a. - wordt gecombineerd met trametinib een anti-PD medicijn in fase I studie na hoopvolle resultaten.
- Voorbeeldrapporten van receptoren en DNA testen - biomoleculaire profielen uitgevoerd door Caris Lifesciences - van alvleesklierkanker, hersentumoren, melanomen en longkanker
- Vroege diagnose van kanker is de toekomst en is vaak al mogelijk: zie TED talk
- Xeloda (capecitabine) en fluorouracil (5-FU) kunnen levensbedreigende bijwerkingen veroorzaken bij kankerpatienten met een DPD-deficiëntie. FDA pleit voor testen op genmutaties vooraf aan behandelingen.
- Ziekte van Parkinson: prasinezumab, een monoklonaal antilichaam dat alfa-synucleïne bindt, vertraagt sterk de progressie van de ziekte van Parkinson in vergelijking met patienten die beste zorg kregen
- Algemeen: overzicht van artikelen waarin personal medicine een rol speelt.




 Article Info
Article Info 1,3
1,3
Plaats een reactie ...
Reageer op "POLE mutatie: veel kankerpatienten met erfelijke vormen van kanker hebben naast een P1-ligand een POLE mutatie en reageren goed op immuuntherapie met anti-PD medicijnen - checkpointremmers als pembrolizumab en nivolumab"