Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
27 september 2015: Bron: PLoS One. 2015 Jun 18;10(6):e0130142. doi: 10.1371/journal.pone.0130142. eCollection 2015.
De laatste jaren vindt er een enorme ontwikkeling plaats met zogeheten anti-PD medicijnen (anti programmed death) ook wel checkpoint remmers genoemd, die ingezet worden als immuuntherapeutische aanpak, zoals de bekendste nivolumab, Pembrolizumab en atezolimab voorheen MPDL3280A geheten. Maar er zijn veel meer anti-PD medicijnen in studies actief. Ik denk persoonlijk dat met deze anti-PD medicijnen een heleboel kanker onder controle kan worden gebracht omdat het werkt als een vorm van immuuntherapie waarbij het apoptose mechanisme (zelfdoding van de cel) wordt hersteld. Waardoor beschadigde cellen of cellen die hun werk hebben gedaan op een natuurlijke manier worden opgeruimd.
Ialiaanse onderzoekers hebben onderzocht of er nog verschil zit in effectiviteit en kans van aanslaan van deze medicijnen of een patiënt de zogeheten Ligand-1 receptorenstatus / DNA mutatie heeft.
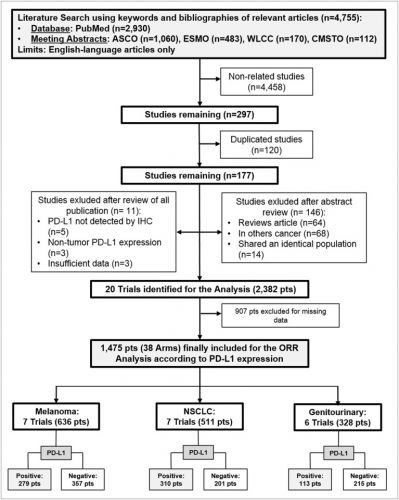
Hieronder de grafiek welke studies bij welke vorm van kanker zij de data hebben verzameld. In dit geval bij melanomen, longkanker en spijsverteringskanker omdat daarin het meest bekend is van studies, maar anti-PD medicijnen werken in principe bij alle vormen van kanker met solide tumoren.
Zij ontdekten dat er wel enig verschil in zit in de kans van succes maar ook kankerpatiënten zonder de Ligand-1 receptorstatus reageren vaak goed op anti-PD medicijnen. Het is ook goed te weten dat deze anti-PD medicijnen tot nu toe ingezet worden bij mensen die al eerder meerdere behandelingen hebben gehad. Interessant zou natuurlijk zijn om deze anti-PD medicijnen in te zetten als immuuntherapie na de eerste diagnose.
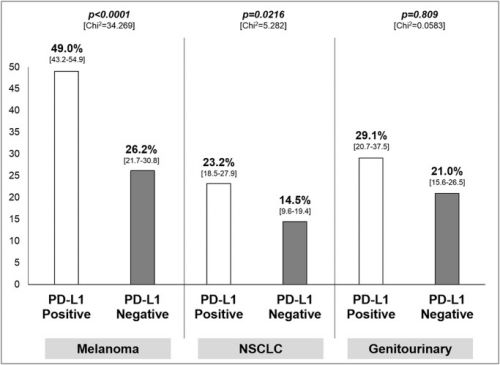
Hier de grafiek van het verschil in Ligand-1 status:
Het is te veel om het studieversalg te vertalen. Zoek in onze zoekmachine op genoemde medicijnen voor studies. Het volledige studierapport: Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers is gratis in te zien met mooie overzichtsgrafieken en interessante referentielijst van studies met ani-PD medicijnen.
Hier het abstract van de studie:
three antibodies provide a significant differential effThe predictive value of PD-L1 on tumor cells seems to be more robust for anti-PD-1 antibody (nivolumab and pembrolizumab), and in the context of advanced melanoma and NSCLC
Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers.
Author information
- 1Medical Oncology, University of Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
- 2Medical Oncology, Regina Elena National Cancer Institute, Roma, Italy.
- 3Department of Pathology and Diagnostic, University of Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
- 4Agenzia Italiana del Farmaco (AIFA), Roma, Italy.
- 5Biostatistics, Regina Elena National Cancer Institute, Roma, Italy.
- 6Department of Pathology and Diagnostic, University of Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy; ARC-NET Center for Applied Research on Cancer, Verona, Italy.
Abstract
BACKGROUND:
The potential predictive role of programmed death-ligand-1 (PD-L1) expression on tumor cells in the context of solid tumor treated with checkpoint inhibitors targeting the PD-1 pathway represents an issue for clinical research.
METHODS:
Overall response rate (ORR) was extracted from phase I-III trials investigating nivolumab, pembrolizumab and MPDL3280A for advanced melanoma, non-small cell lung cancer (NSCLC) and genitourinary cancer, and cumulated by adopting a fixed and random-effect model with 95% confidence interval (CI). Interaction test according to tumor PD-L1 was accomplished. A sensitivity analysis according to adopted drug, tumor type, PD-L1 cut-off and treatment line was performed.
RESULTS:
Twenty trials (1,475 patients) were identified. A significant interaction (p<0.0001) according to tumor PD-L1 expression was found in the overall sample with an ORR of 34.1% (95% CI 27.6-41.3%) in the PD-L1 positive and 19.9% (95% CI 15.4-25.3%) in the PD-L1 negative population. ORR was significantly higher in PD-L1 positive in comparison to PD-L1 negative patients for nivolumab and pembrolizumab, with an absolute difference of 16.4% and 19.5%, respectively. A significant difference in activity of 22.8% and 8.7% according to PD-L1 was found for melanoma and NSCLC, respectively, with no significant difference for genitourinary cancer.
CONCLUSION:
Overall, the three antibodies provide a significant differential effect in terms of activity according to PD-L1 expression on tumor cells. The predictive value of PD-L1 on tumor cells seems to be more robust for anti-PD-1 antibody (nivolumab and pembrolizumab), and in the context of advanced melanoma and NSCLC.
- PMID:
- 26086854
- [PubMed - in process]
- PMCID:
- PMC4472786
-
References
1. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. [PubMed]2. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. [PubMed]3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239 [PubMed]4. Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer cell. 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001 [PMC free article] [PubMed]5. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466 [PMC free article] [PubMed]6. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–9. doi: 10.1056/NEJMe1205943 [PubMed]7. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690 [PMC free article] [PubMed]8. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015. [Epub ahead of print] [PubMed]9. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2 [PubMed]10. Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8 [PubMed]11. Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–65. doi: 10.1016/S1470-2045(15)70054-9 [PubMed]12. Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199–206. doi: 10.1200/JCO.2012.48.3685 [PubMed]13. Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. doi: 10.1016/S1470-2045(13)70551-5 [PMC free article] [PubMed]14. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2014;33(13):1430–7 doi: 10.1200/JCO.2014.59.0703 [PubMed]15. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi: 10.1038/nature13904 [PubMed]16. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694 [PMC free article] [PubMed]17. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133 [PMC free article] [PubMed]18. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105 [PubMed]19. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369 [PubMed]20. Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology. 2014;28 Suppl 3:39–48. [PubMed]21. Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117(10):2192–201. doi: 10.1002/cncr.25747 [PubMed]22. Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5. [PubMed]23. Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: A meta-analysis. Eur J Sur Oncol. 2015;41(4):450–456 [PubMed]24. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082 [PubMed]25. Pignon JP, Hill C. Meta-analyses of randomised clinical trials in oncology. Lancet Oncol. 2001;2(8):475–82. [PubMed]26. Bria E, Gralla RJ, Raftopoulos H, Cuppone F, Milella M, Sperduti I, et al. Magnitude of benefit of adjuvant chemotherapy for non-small cell lung cancer: Meta-analysis of randomized clinical trials. Lung Cancer. 2009;63(1):50–7. doi: 10.1016/j.lungcan.2008.05.002 [PubMed]27. Higgins JPT, Green S. Cochrane handbook for Systematic Reviews of intervention 4.2.6 [updated sep 2006] The Cochrane Library. Issue 4 Chichester, UK: John Wiley & Sons, Ltd; 2006.28. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. [PubMed]29. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 [PMC free article] [PubMed]30. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219 [PMC free article] [PubMed]31. Pilotto S, Di Maio M, Peretti U, Kinspergher S, Brunelli M, Massari F, et al. Predictors of outcome for patients with lung adenocarcinoma carrying the epidermal growth factor receptor mutation receiving 1st-line tyrosine kinase inhibitors: Sensitivity and meta-regression analysis of randomized trials. Crit Rev Oncol Hematol. 2014;90(2):135–45. doi: 10.1016/j.critrevonc.2013.11.005 [PubMed]32. Choueiri T, Fishman MN, Escudier B, Kim JJ, Kluger H, Stadler WM, et al. 1051PDImmunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (MRCC): biomarker-based results from a randomized clinical trial. Ann Oncol. 2014;25(suppl 4):iv362.33. Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). ASCO Meeting Abstracts. 2013;31(15_suppl):9010.34. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011 [PubMed]35. Hodi FS, Sznol M, Kluger HM, McDermott DF, Carvajal RD, Lawrence DP, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. ASCO Meeting Abstracts. 2014;32(15_suppl):9002.36. Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. ASCO Meeting Abstracts. 2014;32(15_suppl):3005.37. Rizvi NA, Garon EB, Patnaik A, Gandhi L, Leighl NB, Balmanoukian AS, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts. 2014;32(15_suppl):8007.38. Sznol M, Kluger HM, Callahan MK, Postow MA, Gordon RA, Segal NH, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). ASCO Meeting Abstracts. 2014;32(15_suppl):LBA9003.39. Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–8. doi: 10.1200/JCO.2013.51.4802 [PMC free article] [PubMed]40. Hammers H, Plimack ER, Infante JR, Ernstoff M, Rini BI, McDermott DF, et al. 1050OPhase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (MRCC). Ann Oncol. 2014;25(suppl 4):iv361–iv2.41. Plimack ER, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez EJ, et al. LBA23A Phase 1B study of pembrolizumab (pembro; mk-3475) in patients (pts) with advanced urothelial tract cancer. Ann Oncol. 2014;25(suppl 4).42. Cho DC, Sosman JA, Sznol M, Gordon MS, Hollebecque A, Hamid O, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC). ASCO Meeting Abstracts. 2013;31(15_suppl):4505.43. Rizvi NA, Shepherd FA, Antonia SJ, Brahmer JR, Chow LQ, Goldman J, et al. First-Line Monotherapy With Nivolumab (Anti-PD-1; BMS-936558, ONO-4538) in Advanced Non-Small Cell Lung Cancer (NSCLC): Safety, Efficacy, and Correlation of Outcomes With PD-L1 Status. Int J Radiation Oncol Biol Phys. 2014;90(5):S31.44. Antonia SJ, Gettinger S, Goldman J, Chow LQ, Juergens R, Borghaei H, et al. Safety and Efficacy of First-Line Nivolumab (Anti-PD-1; BMS-936558, ONO-4538) and Ipilimumab in Non-Small Cell Lung Cancer (NSCLC). Int J Radiation Oncol Biol Phys. 2014;90(5):S32–S3.45. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015. [Epub ahead of print] [PubMed]46. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med. 2015. [Epub ahead of print] [PubMed]47. Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–56. [PubMed]48. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015. [Epub ahead of print] [PubMed]49. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954 [PMC free article] [PubMed]50. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi: 10.1158/1078-0432.CCR-13-3271 [PMC free article] [PubMed]51. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988 [PMC free article] [PubMed]52. Wolchok JD, Chan TA. Cancer: Antitumour immunity gets a boost. Nature. 2014;515(7528):496–8. doi: 10.1038/515496a [PubMed]53. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6. doi: 10.1038/nature14001 [PubMed]54. Boussiotis VA. Somatic mutations and immunotherapy outcome with CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2230–2. doi: 10.1056/NEJMe1413061 [PMC free article] [PubMed]55. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498 [PMC free article] [PubMed]56. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348 [PubMed]57. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med. 2015. [Epub ahead of print] [PubMed]
Articles from PLoS ONE are provided here courtesy of Public Library of Science
Gerelateerde artikelen
- 6 nieuwe doorbraken in de strijd tegen kanker worden gepresenteerd door het World Economic Forum met bijbehorende video
- 90 procent van mensen met uitgezaaide kanker heeft meerdere DNA afwijkingen. Slechts 5 procent kreeg ook optimale behandeling daarvoor.
- Antibiotica binnen een maand vooraf aan immuuntherapie met anti-PD medicijnen geeft veel slechtere resultaten op overall overleving dan zonder antibiotica bij verschillende vormen van primaire kanker.
- Anti-PD medicijnen zoals nivolumab, Pembrolizumab en atezolizumab gegeven als immuuntherapie geven zeer goede resultaten bij verschillende vormen van kanker met solide tumoren, zelfs zonder Ligand-1 receptorstatus copy 1
- Bacterien in uitzaaiingen van kankerpatienten zijn door Nederlandse onderzoekers in beeld gebracht en in een gedetailleerde catalogus opgeslagen
- Behandelen van kanker verschuift steeds meer van chemotherapie naar biologische behandelingen, gerichte therapie waaronder immuuntherapie met gemoduleerde virussen die de minste bijwerkingen geven
- Biomarkers zoals PD-L1, CD163+ en NRAS mutaties en gegevens zoals uitzaaiingen later ontstaan bepalen kans van effectiviteit van immuuntherapie met anti PD medicijnen bij melanomen
- Bloedtesten, een overzicht van recent gepubliceerde resultaten van verschillende bloedtesten
- CRISPR-Cas kan veranderingen in bacterien bewerkstelligen en via bewerkte bacterien verspreiden om zo resistentie van antibiotica te veranderen in opnieuw werkende antibiotica.
- CHRISPR-CAS9 infuus blijkt genezende behandeling voor erfelijke aandoening angio-oedeem, aldus tussenresultaten van internationale studie met Nederlandse deelname.
- CLEVER studie: Waarom kanker jaren later kan terugkeren - en hoe dit te voorkomen. CLEVER studie bij borstkankerpatienten bewijst dat een recidief is te voorkomen door tumorcellen in beenmerg te behandelen.
- De biologische processen waarom en hoe kankercellen uitzaaien wordt beter begrepen, tumorcellen vroeger ontdekt en lijkt ook steeds beter te behandelen
- De huidige staat van moleculair testen in het behandelen van kankerpatienten met solide tumoren. Een uitstekend overzichtsartikel met de nieuwste ontwikkelingen over RNA, DNA en eiwitten anno 2019
- Diagnosetest PERCEPTION via AI - Kunstmatige Intelligentie ontwikkeld en met hulp van single-cell RNA-sequencing voorspelt nauwkeurig of een specifiek medicijn van de kankerpatient zal aanslaan of resistent zal zijn.
- DRUP studie geeft bij 37 procent van de patienten alsnog een therapeutisch effect met 6 procent CR en 14 procent PR en 17 procent stabiele ziekte
- EMA: Veel nieuwe kankermedicijnen in de EU hebben geen bewezen toegevoegde waarde blijkt uit Nederlandse studie naar goedgekeurde kankermedicijnen door het Europees Geneesmiddelenbureau (EMA).
- Erfelijkheid van kanker hangt vaak af van specifieke afwijkende genencombinaties in DNA onderzoek en eiwitexpressie blijkt uit groot Whole Exome Sequencing onderzoek via de Biobank van de UK.
- ESMO - European Society for Medical Oncology heeft een gids uitgegeven voor patienten over hoe personalised medicine werkt en stand van zaken
- FDA ondersteunt onderzoek naar personalised medicine op basis van mutaties ongeacht in welk lichaamsdeel de kanker zich het eerst openbaart.
- Genetisch onderzoek via Germline testen (kiembaan testen) werd in periode 2013 tot 2019 in Georgie en Californie bij slechts 7 procent gedaan onder 1 369 602 patienten met twee jaar kanker.
- Genetische mutatie ontdekt die kans van slagen van immuuntherapie bij alle vormen van kanker naar 100 procent zou kunnen brengen
- Geneesmiddel (ARS1620) verandert kankergen (KRAS mutatie) dat kwaadaardige tumoren beschermt tegen immuunsysteem in een doelwit voor immuunsysteem en helpt immuuntherapie kankercellen te elimineren
- Gentherapie zoals Chrispr-cas en base-editors zijn zeer succesvol bij erfelijke ziekten waaronder ook vormen van kanker zoals sikkelcelziekte
- Gerichte behandelingen met Aurora kinaseremmers geven soms uitstekende resultaten bij veel vormen van kanker. Een reviewstudie
- Immuunafwijkingen bij kankerpatienten gerelateerd aan infecties voortijdig ontdekken zouden in behandelingen van kanker sterven aan kanker met 25 procent of meer kunnen voorkomen
- Immuuntherapie met HER2-gerichte CT-0508 (CAR-Macrofaag therapie) geeft bij solide tumoren van verschillende vormen van kanker met HER2 positieve expressie hoopvolle resultaten
- Immuuntherapie met pembrolizumab bij patiënten met verschillende vormen van uitgezaaide kanker met hoge microsatellietinstabiliteit (MSI-H) en DNA-mismatch-reparatie-deficiënte (dMMR) geeft uitstekende en duurzame resultaten op overall overleving
- Immuuntherapie met nivolumab zorgt voor duurzame en sterk verbeterde overall overleving bij verschillende vormen van kanker, melanomen, longkanker en nierkanker copy 1
- Interleukin-15 speelt een cruciale rol wanneer gegeven samen met immuuntherapie voor verschillende vormen van kanker met solide tumoren en biedt veelbelovende mogelijkheden voor verbeterde behandelingen
- Internationale groep van 180 wetenschappers stelt rapport op hoe en met welke niet-toxische middelen - voedingsstoffen de effectiviteit te verbeteren, recidieven te voorkomen en de bijwerkingen te verminderen van personalised medicine
- Irina Kareva gebruikt wiskundige modellen die de dynamiek van kanker beschrijven, met het doel nieuwe geneesmiddelen te ontwikkelen die gericht zijn op tumoren.
- Kanker-actueel kan en wil helpen - begeleiden bij aanvragen van een volledig biomoleculair receptorenonderzoek en genenonderzoek
- Erfelijkheid: Kanker is vaak domme pech stelt prof. dr. Nicoline Hoogerbrugge van het Radboudumc en de Radboud Universiteit in een interview in de Stentor en Algemeen Dagblad
- Kankermedicijnen geven in de klinische praktijk veel minder effect dan uit de studies van farmaceutische bedrijven is aangetoond. Maar zijn wel ontzettend duur.
- Kankerremmende eiwitten kunnen bij mutatie die gen uitschakelt veranderen van kankerremmend in stimulerend, ontdekten Nederlandse onderzoekers
- Larotrectinib geeft bijzonder goede resultaten (76 procent respons met 12 procent complete remissies) bij alle vormen van solide tumoren met een positieve TRK Fusion mutatie
- Larotrectinib: Met de goedkeuring van Larotrectinib op basis van 1 specifieke afwijking en niet op basis van primaire tumor zorgt de FDA voor een doorbraak in het behandelen van kanker
- Lenvatinib Plus Pembrolizumab bij patiënten met inoperabele gevorderde nierkanker, buikvlieskanker, melanomen en andere gevorderde kanker met solide tumoren geeft uitstekende resultaten met meer dan de helft remissies van 50 procent of meer copy 1
- Medicijnen voorschrijven op basis van DNA profiel van de patient voorkomt 30 procent minder bijwerkingen blijkt uit internationale studie onder leiding van LUMC Leiden
- Moleculaire schakelaar verandert kankercellen in weer normale cellen en zou genezende aanpak van kanker kunnen betekenen
- MSC-1 een medicijn dat de groei van de kankerstamcellen afremt door LIF blokkade en immuunsysteem activeert laat spectaculair goede resultaten zien in fase I studie.
- Mytomorrows breidt aanbod aan experimentele medicijnen voor kankerpatienten uit met 11 nieuwe nog niet geregistreerde medicijnen en stelt deze beschikbaar voor uitbehandelde kankerpatienten
- Nederland betaalt veel meer voor kankermedicijnen, soms tot 50 procent of meer, dan andere landen blijkt uit vergelijkend onderzoek tussen 18 landen copy 1
- NCI-MATCH-studie toont aan dat een biomoleculaire analyse - DNA en receptorenonderzoek - belangrijk is in hoe een kankerpatient te behandelen.
- Nieuw medicijn - PD-0332991 - stopt groei hersentumoren Glioblastoom in dierproeven. Zodra gestopt werd met dit medicijn gingen de tumoren weer groeien. Fase I studie bij 33 patienten met nierkanker en lymfklierkanker bevestigt veiligheid van dit middel
- Nieuwe, dure kankermedicijnen zijn voortaan sneller beschikbaar door het Drug Access Protocol (DAP) dat is ontwikkeld door oncologen, verzekeraars en Zorginstituut Nederland
- Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie copy 1
- Overzicht van studies met medicijnen en behandelingen om tumoren met KRAS mutaties aan te pakken. Vooral combinatiebehandelingen zijn veelbelovend.
- PI3K/AKT/mTOR pathway speelt cruciale rol in apoptose proces, DNA herstel, metabolisme in de cel en angiogenese.
- Pembrolizumab - Keytruda geeft bij solide tumoren van verschillende oorsprong 21 procent complete remissies en 53 procent gedeeltelijke remissies.
- Personalised medicine door receptorenonderzoek geeft veel betere resultaten in fase 1 studies dan experimenteel onderzoek zonder receptorenonderzoek
- Prof. Bernards over de doorbraak bij darmkanker met Kras mutatie en bij melanomen met BRAF mutatie in DWDD van donderdag 27 maart 2014
- POLE mutatie: veel kankerpatienten met erfelijke vormen van kanker hebben naast een P1-ligand een POLE mutatie en reageren goed op immuuntherapie met anti-PD medicijnen - checkpointremmers als pembrolizumab en nivolumab
- Radiotherapeutisch stimulerend middel NBTXR3 geeft in combinatie met anti-PD-1 medicijnen alsnog uitstekende resultaten bij patiënten die ziekteprogressie lieten zien ongeacht eerdere behandeling met anti-PD-1 medicijnen
- Rezatapopt, een p53-reactivator gericht op de p53 Y220C-mutatie, geeft in veiligheidstudie hoopvolle resultaten bij zwaarvoorbehandelde kankerpatienten met solide tumoren
- Rozlytrek (entrectinib), een tyrosine kinase remmer, goedgekeurd door FDA als medicijn voor solide tumoren met NTRK (neurotrophic tyrosine receptor kinase) gene fusion. Dit is 3e goedgekeurde medicijn op basis van mutatie.
- Stamceltherapie succesvol toegepast bij vrouw met diabetes type 1. Zij hoeft nu al een jaar geen insuline meer te spuiten.
- Tweede primaire vorm van kanker bij een kankerpatient wordt steeds vaker bekend bij de diagnose (2 tot 17 procent) door betere diagnose technieken en verfijnder biomoleculair onderzoek
- Tumorindeling mede aan de hand van biomarkers - biomoleculaire profielen is nodig en zal behandelingen sterk veranderen voor veel kankerpatiënten. Van 10 procent nu tot 50 procent straks. Aldus grote studie van het TOGA
- Vaccin tegen KRAS positief gemuteerde vormen van kanker - darmkankers en longkanker o.a. - wordt gecombineerd met trametinib een anti-PD medicijn in fase I studie na hoopvolle resultaten.
- Voorbeeldrapporten van receptoren en DNA testen - biomoleculaire profielen uitgevoerd door Caris Lifesciences - van alvleesklierkanker, hersentumoren, melanomen en longkanker
- Vroege diagnose van kanker is de toekomst en is vaak al mogelijk: zie TED talk
- Xeloda (capecitabine) en fluorouracil (5-FU) kunnen levensbedreigende bijwerkingen veroorzaken bij kankerpatienten met een DPD-deficiëntie. FDA pleit voor testen op genmutaties vooraf aan behandelingen.
- Ziekte van Parkinson: prasinezumab, een monoklonaal antilichaam dat alfa-synucleïne bindt, vertraagt sterk de progressie van de ziekte van Parkinson in vergelijking met patienten die beste zorg kregen
- Algemeen: overzicht van artikelen waarin personal medicine een rol speelt.



Plaats een reactie ...
Reageer op "Anti-PD medicijnen zoals nivolumab, Pembrolizumab en atezolizumab gegeven als immuuntherapie geven zeer goede resultaten bij verschillende vormen van kanker met solide tumoren, zelfs zonder Ligand-1 receptorstatus copy 1"