Helpt u ona aan 500 donateurs?
16 januari 2018: Lees ook dit artikel
25 december 2017: Hier het originele volledige studierapport van onderstaande studie, waarin pembrolizumab (Keytruda) bij 11 verschillende primaire vormen van kanker met solide tumoren met MSI mutatie voor spectaculaire resultaten zorgt (zie onder grafiek verslag daarvan d.d. 11 juni 2017, abstract staat onderaan artikel).
Deze studie is door Sciencemagazine uitgeroepen als een van de twee beste wetenschappelijke doorbraken van 2017.
Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade
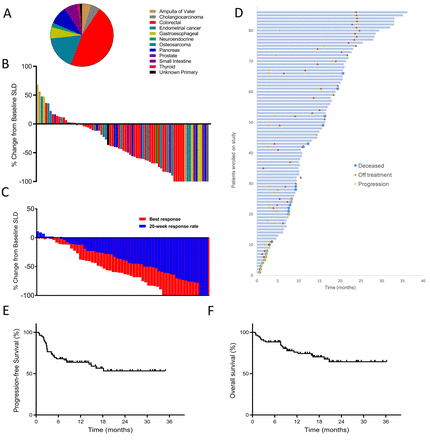
Fig. 1
Patient survival and clinical response to Pembrolizumab across 12 different tumor types with mismatch repair deficiency. (A) Tumor types across 86 patients. (B) Waterfall plot of all radiographic responses across 12 different tumor types at 20 weeks. Tumor responses were measured at regular intervals and values show the best fractional change of the sum of longest diameters (SLD) from the baseline measurements of each measurable tumor. (C) Confirmed radiographic objective responses at 20 weeks in blue compared to the best radiographic responses in the same patients in red. The mean time to the best radiographic response was 28 weeks. (D) Swimmer plot showing survival for each patient with mismatch repair deficient tumors, indicating death, progression and time off therapy. (E) Kaplan-Meier estimates of progression-free survival and (F) overall patient survival.
11 juni 2017: Bron: Science en ASCO 2017
Immuuntherapie met pembrolizumab (Keytruda) geeft bij solide tumoren met de juiste mutatie / eiwitexpressie vanuit 12 verschillende primaire tumoren uitstekende resultaten met 53 procent gedeeltelijke remissies en 21 procent complete remissies. En progressievrije tijd en overall overleving is nog lang niet bereikt. .png)
Keytruda is al goedgekeurd voor melanomen en borstkanker maar blijkt nu dus ook effectief te zijn voor kanker vanuit andere primaire vormen van kanker. Waaronder alvleesklierkanker, prostaatkanker, darmkanker enz.
De primaire vorm doet er niet meer toe, als de tumorcellen een bepaalde expressie hebben, er sprake is van een bepaalde mutatie, dan is de kans heel groot dat pembrolizumab aanslaat. In het abstract staat er niet bij of de afwijking die gevraagd wordt ook de P1-ligand mutatie is. Bij andere anti-PD medicijnen, zoals atezolizumab of avelumab maakt het meestal niet zo heel veel uit of de mutatie P1-ligand wel verhoogd is of niet.
Uit het abstract van de studie:
We have expanded this study to now evaluate efficacy of PD-1 blockade in patients with advanced MMR-deficient cancers across 12 different tumor types. Objective radiographic responses were observed in 53% of patients and complete responses were achieved in 21% of patients. Responses were durable with median progression-free and overall survival still not reached.
In een artikel in het Algemeen Dagblad over deze studie zegt dr. Jan Schellens van het Anthonie van Leeuwenhoek Ziekenhuis het volgende:
Immunotherapie
Jan Schellens, medisch oncoloog en geneesmiddelendeskundige van het Antoni van Leeuwenhoek in Amsterdam, legt uit hoe Keytruda precies werkt. ,,Het medicijn wordt gebruikt bij een type behandeling die wij immuuntherapie noemen. Het helpt het immuunsysteem om tumoren aan te vallen. Vanuit het afweersysteem worden T-cellen geproduceerd. Deze worden extra geactiveerd als ze in aanraking komen met de stof pembrolizumab, zodat ze kankercellen beter herkennen en kunnen ruimen.''
,,Het unieke aan deze toepassing is dat de patiënten geselecteerd kunnen worden voor behandeling met pembrolizumab aan de hand van een afwijkend eiwit in het kankerweefsel'', gaat hij verder. ,,Dat is nog nooit eerder zo gedaan. Waar we voorheen voornamelijk keken naar het soort kanker - dat kan zijn darmkanker, alvleesklierkanker, slokdarmkanker, borstkanker, maagkanker, etc. – kunnen we nu op een heel andere manier patiënten identificeren en behandelen.''>>>>>>>>Lees verder het hele artikel in het AD
Een andere studie met min of meer dezelfde insteek is deze: Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors waarvan het studierapport volledig gratis is in te zien. Met interessante referentielijst. Zie onderaan artikel
De studie: Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade is tegen betaling verkrijgbaar.
Hier het abstract van deze studie:
the large proportion of mutant neoantigens in MMR-deficient cancers make them sensitive to immune checkpoint blockade, regardless of the cancers’ tissue of origin
+ See all authors and affiliations
Abstract
The genomes of cancers deficient in mismatch repair (MMR) contain exceptionally high numbers of somatic mutations. In a proof-of-concept study, we previously showed that colorectal cancers with MMR deficiency were sensitive to immune checkpoint blockade with anti-PD-1 antibodies. We have expanded this study to now evaluate efficacy of PD-1 blockade in patients with advanced MMR-deficient cancers across 12 different tumor types. Objective radiographic responses were observed in 53% of patients and complete responses were achieved in 21% of patients. Responses were durable with median progression-free and overall survival still not reached. Functional analysis in a responding patient demonstrated rapid in vivo expansion of neoantigen-specific T cell clones that were reactive to mutant neopeptides found in the tumor. These data support the hypothesis that the large proportion of mutant neoantigens in MMR-deficient cancers make them sensitive to immune checkpoint blockade, regardless of the cancers’ tissue of origin.
Pembrolizumab at both 2 and 10 mg/kg Q2W was well tolerated in Japanese patients with advanced solid tumors and showed encouraging anti-tumor activity against melanoma and NSCLC.
Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors
Toshio Shimizu, Takashi Seto, Fumihiko Hirai, Mitsuhiro Takenoyama, Kaname Nosaki, Junji Tsurutani, Hiroyasu Kaneda, Tsutomu Iwasa, Hisato Kawakami, Kazuo Noguchi, Takashi Shimamoto, and Kazuhiko Nakagawa
Takashi Seto, Fumihiko Hirai, Mitsuhiro Takenoyama, Kaname Nosaki, Junji Tsurutani, Hiroyasu Kaneda, Tsutomu Iwasa, Hisato Kawakami, Kazuo Noguchi, Takashi Shimamoto, and Kazuhiko Nakagawa
Summary
Background This phase I study evaluated the safety and tolerability, pharmacokinetics and pharmacodynamics, immunogenicity, and antitumor activity of pembrolizumab in Japanese patients with advanced solid tumors. Methods Following an initial dose and a 28-day rest (cycle 1), pembrolizumab was administered as an intravenous infusion at escalating doses (2 or 10 mg/kg) every 2 weeks (Q2W) until disease progression or unacceptable toxicity. Adverse events (AEs) were assessed using CTCAE v4.0, and tumor response was assessed using both RECIST v1.1 and immune-related response criteria (irRC). Full pharmacokinetic sampling was performed during cycle 1. Results Three patients received pembrolizumab at 2.0 mg/kg and seven at 10 mg/kg. No dose-limiting toxicities were observed during cycle 1. Eighty percent of patients experienced drug-related AEs (mostly grade 1 or 2); the most common drug-related AEs were nausea, malaise, pyrexia, and aspartate aminotransferase/alanine transaminase (AST/ALT) elevations (n = 2 each). No drug-related grade 4 or 5 AEs occurred. Immune-related AEs comprised grade 3 ALT elevation (n = 1), grade 3 AST elevation (n = 1), grade 1 pneumonitis (n = 1), and grade 1 thyroid-stimulating hormone elevation (n = 1). The safety and pharmacokinetic profiles of Japanese patients were similar to those previously reported for Caucasian patients. A partial tumor response was observed in one patient with non-small-cell lung cancer (NSCLC) and in one patient with melanoma. Conclusions Pembrolizumab at both 2 and 10 mg/kg Q2W was well tolerated in Japanese patients with advanced solid tumors and showed encouraging anti-tumor activity against melanoma and NSCLC.
References
Gerelateerde artikelen
- 6 nieuwe doorbraken in de strijd tegen kanker worden gepresenteerd door het World Economic Forum met bijbehorende video
- 90 procent van mensen met uitgezaaide kanker heeft meerdere DNA afwijkingen. Slechts 5 procent kreeg ook optimale behandeling daarvoor.
- Antibiotica binnen een maand vooraf aan immuuntherapie met anti-PD medicijnen geeft veel slechtere resultaten op overall overleving dan zonder antibiotica bij verschillende vormen van primaire kanker.
- Anti-PD medicijnen zoals nivolumab, Pembrolizumab en atezolizumab gegeven als immuuntherapie geven zeer goede resultaten bij verschillende vormen van kanker met solide tumoren, zelfs zonder Ligand-1 receptorstatus copy 1
- Bacterien in uitzaaiingen van kankerpatienten zijn door Nederlandse onderzoekers in beeld gebracht en in een gedetailleerde catalogus opgeslagen
- Behandelen van kanker verschuift steeds meer van chemotherapie naar biologische behandelingen, gerichte therapie waaronder immuuntherapie met gemoduleerde virussen die de minste bijwerkingen geven
- Biomarkers zoals PD-L1, CD163+ en NRAS mutaties en gegevens zoals uitzaaiingen later ontstaan bepalen kans van effectiviteit van immuuntherapie met anti PD medicijnen bij melanomen
- Bloedtesten, een overzicht van recent gepubliceerde resultaten van verschillende bloedtesten
- CHRISPR-CAS9 infuus blijkt genezende behandeling voor erfelijke aandoening angio-oedeem, aldus tussenresultaten van internationale studie met Nederlandse deelname.
- CLEVER studie: Waarom kanker jaren later kan terugkeren - en hoe dit te voorkomen. CLEVER studie bij borstkankerpatienten bewijst dat een recidief is te voorkomen door tumorcellen in beenmerg te behandelen.
- De biologische processen waarom en hoe kankercellen uitzaaien wordt beter begrepen, tumorcellen vroeger ontdekt en lijkt ook steeds beter te behandelen
- De huidige staat van moleculair testen in het behandelen van kankerpatienten met solide tumoren. Een uitstekend overzichtsartikel met de nieuwste ontwikkelingen over RNA, DNA en eiwitten anno 2019
- Diagnosetest PERCEPTION via AI - Kunstmatige Intelligentie ontwikkeld en met hulp van single-cell RNA-sequencing voorspelt nauwkeurig of een specifiek medicijn van de kankerpatient zal aanslaan of resistent zal zijn.
- DRUP studie geeft bij 37 procent van de patienten alsnog een therapeutisch effect met 6 procent CR en 14 procent PR en 17 procent stabiele ziekte
- EMA: Veel nieuwe kankermedicijnen in de EU hebben geen bewezen toegevoegde waarde blijkt uit Nederlandse studie naar goedgekeurde kankermedicijnen door het Europees Geneesmiddelenbureau (EMA).
- Erfelijkheid van kanker hangt vaak af van specifieke afwijkende genencombinaties in DNA onderzoek en eiwitexpressie blijkt uit groot Whole Exome Sequencing onderzoek via de Biobank van de UK.
- ESMO - European Society for Medical Oncology heeft een gids uitgegeven voor patienten over hoe personalised medicine werkt en stand van zaken
- FDA ondersteunt onderzoek naar personalised medicine op basis van mutaties ongeacht in welk lichaamsdeel de kanker zich het eerst openbaart.
- Genetisch onderzoek via Germline testen (kiembaan testen) werd in periode 2013 tot 2019 in Georgie en Californie bij slechts 7 procent gedaan onder 1 369 602 patienten met twee jaar kanker.
- Genetische mutatie ontdekt die kans van slagen van immuuntherapie bij alle vormen van kanker naar 100 procent zou kunnen brengen
- Geneesmiddel (ARS1620) verandert kankergen (KRAS mutatie) dat kwaadaardige tumoren beschermt tegen immuunsysteem in een doelwit voor immuunsysteem en helpt immuuntherapie kankercellen te elimineren
- Gentherapie zoals Chrispr-cas en base-editors zijn zeer succesvol bij erfelijke ziekten waaronder ook vormen van kanker zoals sikkelcelziekte
- Gerichte behandelingen met Aurora kinaseremmers geven soms uitstekende resultaten bij veel vormen van kanker. Een reviewstudie
- Immuunafwijkingen bij kankerpatienten gerelateerd aan infecties voortijdig ontdekken zouden in behandelingen van kanker sterven aan kanker met 25 procent of meer kunnen voorkomen
- Immuuntherapie met HER2-gerichte CT-0508 (CAR-Macrofaag therapie) geeft bij solide tumoren van verschillende vormen van kanker met HER2 positieve expressie hoopvolle resultaten
- Immuuntherapie met pembrolizumab bij patiënten met verschillende vormen van uitgezaaide kanker met hoge microsatellietinstabiliteit (MSI-H) en DNA-mismatch-reparatie-deficiënte (dMMR) geeft uitstekende en duurzame resultaten op overall overleving
- Immuuntherapie met nivolumab zorgt voor duurzame en sterk verbeterde overall overleving bij verschillende vormen van kanker, melanomen, longkanker en nierkanker copy 1
- Interleukin-15 speelt een cruciale rol wanneer gegeven samen met immuuntherapie voor verschillende vormen van kanker met solide tumoren en biedt veelbelovende mogelijkheden voor verbeterde behandelingen
- Internationale groep van 180 wetenschappers stelt rapport op hoe en met welke niet-toxische middelen - voedingsstoffen de effectiviteit te verbeteren, recidieven te voorkomen en de bijwerkingen te verminderen van personalised medicine
- Irina Kareva gebruikt wiskundige modellen die de dynamiek van kanker beschrijven, met het doel nieuwe geneesmiddelen te ontwikkelen die gericht zijn op tumoren.
- Kanker-actueel kan en wil helpen - begeleiden bij aanvragen van een volledig biomoleculair receptorenonderzoek en genenonderzoek
- Erfelijkheid: Kanker is vaak domme pech stelt prof. dr. Nicoline Hoogerbrugge van het Radboudumc en de Radboud Universiteit in een interview in de Stentor en Algemeen Dagblad
- Kankermedicijnen geven in de klinische praktijk veel minder effect dan uit de studies van farmaceutische bedrijven is aangetoond. Maar zijn wel ontzettend duur.
- Kankerremmende eiwitten kunnen bij mutatie die gen uitschakelt veranderen van kankerremmend in stimulerend, ontdekten Nederlandse onderzoekers
- Larotrectinib geeft bijzonder goede resultaten (76 procent respons met 12 procent complete remissies) bij alle vormen van solide tumoren met een positieve TRK Fusion mutatie
- Larotrectinib: Met de goedkeuring van Larotrectinib op basis van 1 specifieke afwijking en niet op basis van primaire tumor zorgt de FDA voor een doorbraak in het behandelen van kanker
- Lenvatinib Plus Pembrolizumab bij patiënten met inoperabele gevorderde nierkanker, buikvlieskanker, melanomen en andere gevorderde kanker met solide tumoren geeft uitstekende resultaten met meer dan de helft remissies van 50 procent of meer copy 1
- Medicijnen voorschrijven op basis van DNA profiel van de patient voorkomt 30 procent minder bijwerkingen blijkt uit internationale studie onder leiding van LUMC Leiden
- Moleculaire schakelaar verandert kankercellen in weer normale cellen en zou genezende aanpak van kanker kunnen betekenen
- MSC-1 een medicijn dat de groei van de kankerstamcellen afremt door LIF blokkade en immuunsysteem activeert laat spectaculair goede resultaten zien in fase I studie.
- Mytomorrows breidt aanbod aan experimentele medicijnen voor kankerpatienten uit met 11 nieuwe nog niet geregistreerde medicijnen en stelt deze beschikbaar voor uitbehandelde kankerpatienten
- Nederland betaalt veel meer voor kankermedicijnen, soms tot 50 procent of meer, dan andere landen blijkt uit vergelijkend onderzoek tussen 18 landen copy 1
- NCI-MATCH-studie toont aan dat een biomoleculaire analyse - DNA en receptorenonderzoek - belangrijk is in hoe een kankerpatient te behandelen.
- Nieuw medicijn - PD-0332991 - stopt groei hersentumoren Glioblastoom in dierproeven. Zodra gestopt werd met dit medicijn gingen de tumoren weer groeien. Fase I studie bij 33 patienten met nierkanker en lymfklierkanker bevestigt veiligheid van dit middel
- Nieuwe, dure kankermedicijnen zijn voortaan sneller beschikbaar door het Drug Access Protocol (DAP) dat is ontwikkeld door oncologen, verzekeraars en Zorginstituut Nederland
- Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie copy 1
- Overzicht van studies met medicijnen en behandelingen om tumoren met KRAS mutaties aan te pakken. Vooral combinatiebehandelingen zijn veelbelovend.
- PI3K/AKT/mTOR pathway speelt cruciale rol in apoptose proces, DNA herstel, metabolisme in de cel en angiogenese.
- Pembrolizumab - Keytruda geeft bij solide tumoren van verschillende oorsprong 21 procent complete remissies en 53 procent gedeeltelijke remissies.
- Personalised medicine door receptorenonderzoek geeft veel betere resultaten in fase 1 studies dan experimenteel onderzoek zonder receptorenonderzoek
- Prof. Bernards over de doorbraak bij darmkanker met Kras mutatie en bij melanomen met BRAF mutatie in DWDD van donderdag 27 maart 2014
- POLE mutatie: veel kankerpatienten met erfelijke vormen van kanker hebben naast een P1-ligand een POLE mutatie en reageren goed op immuuntherapie met anti-PD medicijnen - checkpointremmers als pembrolizumab en nivolumab
- Radiotherapeutisch stimulerend middel NBTXR3 geeft in combinatie met anti-PD-1 medicijnen alsnog uitstekende resultaten bij patiënten die ziekteprogressie lieten zien ongeacht eerdere behandeling met anti-PD-1 medicijnen
- Rozlytrek (entrectinib), een tyrosine kinase remmer, goedgekeurd door FDA als medicijn voor solide tumoren met NTRK (neurotrophic tyrosine receptor kinase) gene fusion. Dit is 3e goedgekeurde medicijn op basis van mutatie.
- Stamceltherapie succesvol toegepast bij vrouw met diabetes type 1. Zij hoeft nu al een jaar geen insuline meer te spuiten.
- Tweede primaire vorm van kanker bij een kankerpatient wordt steeds vaker bekend bij de diagnose (2 tot 17 procent) door betere diagnose technieken en verfijnder biomoleculair onderzoek
- Tumorindeling mede aan de hand van biomarkers - biomoleculaire profielen is nodig en zal behandelingen sterk veranderen voor veel kankerpatiënten. Van 10 procent nu tot 50 procent straks. Aldus grote studie van het TOGA
- Vaccin tegen KRAS positief gemuteerde vormen van kanker - darmkankers en longkanker o.a. - wordt gecombineerd met trametinib een anti-PD medicijn in fase I studie na hoopvolle resultaten.
- Voorbeeldrapporten van receptoren en DNA testen - biomoleculaire profielen uitgevoerd door Caris Lifesciences - van alvleesklierkanker, hersentumoren, melanomen en longkanker
- Vroege diagnose van kanker is de toekomst en is vaak al mogelijk: zie TED talk
- Xeloda (capecitabine) en fluorouracil (5-FU) kunnen levensbedreigende bijwerkingen veroorzaken bij kankerpatienten met een DPD-deficiëntie. FDA pleit voor testen op genmutaties vooraf aan behandelingen.
- Ziekte van Parkinson: prasinezumab, een monoklonaal antilichaam dat alfa-synucleïne bindt, vertraagt sterk de progressie van de ziekte van Parkinson in vergelijking met patienten die beste zorg kregen
- Algemeen: overzicht van artikelen waarin personal medicine een rol speelt.




Plaats een reactie ...
Reageer op "Pembrolizumab - Keytruda geeft bij solide tumoren van verschillende oorsprong 21 procent complete remissies en 53 procent gedeeltelijke remissies."