NANOBIOTIX heeft op ASCO 2021 nieuwe gegevens gepubliceerd van hun middel NBTXR3 (merknaam HENSIFY) dat het immuunsysteem versterkt door radiotherapie - bestraling door de inbreng via nanodeeltjes. NBTXR3 wordt alleen gegeven in combinatie met anti-PD-1 medicijnen.
In een nieuwe studie bij patiënten met solide tumoren die ziekteprogressie hadden, zelfs ondanks gebruik van immuuntherapie met anti-PD medicijnen, laat zien dat bij 76,9% van de evalueerbare patiënten (N = 16) afname van tumoromvang en tumoraantal wordt bereikt. 60 procent bereikte minimaal een gedeeltelijke remissie van 50 procent of meer. 1 patiënt bereikte zelfs een complete remissie.
In deze nieuwe studie zijn pembrolizumab en nivolumab gebruikt als anti-PD medicijnen bij longkankerpatiënten en mond- en keelkankerpatienten. Maar er lopen ook studies bij andere vormen van kanker met solide tumoren en andere anti-PD medicijnen.
Zie deze studies al in 2019 gepland:
NBTXR3 is already undergoing testing in several Phase 1/2 trials for the treatment of head and neck cancer (NCT01946867, NCT02901483), liver cancer (NCT02721056), rectal cancer (NCT02465593), and prostate cancer (NCT02805894).
An additional Phase 1/2 trial (NCT03589339) will also test the treatment in combination with a PD-1 inhibitor — Opdivo (nivolumab) or Keytruda (pembrolizumab) — in patients with advanced head and neck squamous cell carcinoma or metastatic non-small cell lung cancer.
Nu dus deze publicatie van de laatste studie bij longkanker en mond- en keelkanker.
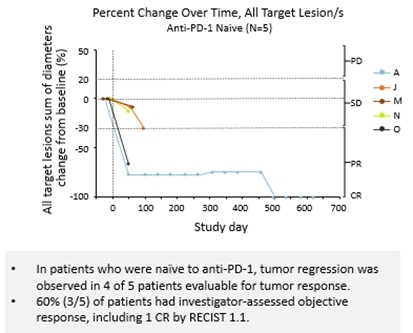
In deze nieuwe studie zijn pembrolizumab en nivolumab gebruikt als anti-PD medicijnen bij longkankerpatiënten en mond- en keelkankerpatienten. Maar er lopen ook studies bij andere vormen vankanker met solide tumoren en andere anti-PD medicijnen. Op deze pagina van de website van NANOBIOTIX staat hoe een an ander is ontwikkeld en een video met uitleg over hoe NBTXR3 precies werkt en wordt toegediend.
Voor sarcomen heeft NANOBIOTIX al in 2014 officiele toestemming van de EMA gekregen op basis van deze studie: NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial
Op ASCO is een abstract gepresenteerd over de nieuwste studie maar businesswire heeft uitgebreidere informatie over deze nieuwe studie. Klik op de titel hieronder.
Abstract in Clinical Oncology staat hier:
https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.15_suppl.2590
Busenesswire artikel is dit:
NANOBIOTIX Reports New Data for Potential First-in-Class Radioenhancer NBTXR3 in Combination With Anti-PD-1 Showing Local or Distant Tumor Regression in 76.9% of Evaluable Patients Regardless of Prior Anti-PD-1 Exposure
Data to be Presented at the 2021 Annual Meeting of the American Society for Clinical Oncology
- Results show NBTXR3 plus radiotherapy could potentially stimulate immune response and convert anti-PD-1 non-responders into responders
- Objective response was observed in 60% of anti-PD-1 naïve patients and 50% of prior non-responders
- Data suggest abscopal effect in some patients (i.e., reduction in non-injected / non-irradiated lesions)
- To date, the overall adverse event profile for the 16 injected patients has not differed from what is expected with radiotherapy or anti-PD-1 agents (head and neck cancer and non-small cell lung cancer primary tumors)
- New readout could provide promising signals for NBTXR3 as a potential pillar of immunotherapy
- Following ASCO, Nanobiotix will host an investor event on Friday, June 11, 2021 at 8:00 am Eastern Time (14:00 Central European Time), to provide an in-depth review of the immunotherapy data with several key opinion leaders including study investigators (Register here)
Priming Immune Response and Immunotherapy Combination in Advanced Cancers
Abstract #2590: A Phase I Study of NBTXR3 Activated by Radiotherapy for Patients with Advanced Cancers Treated with an Anti-PD-1 Therapy
Background
The Nanobiotix phase I study of NBTXR3 activated by radiotherapy for patients with advanced cancers treated with an anti-PD-1 therapy (Study 1100), is a multicenter, open-label, non-randomized phase I dose escalation with dose expansion study to establish the recommended phase II dose (RP2D) of NBTXR3 plus radiotherapy in combination with anti-PD-1 in three (3) cohorts: (i) inoperable locoregional recurrent or recurrent and metastatic head and neck cancer (R/M head and neck squamous cell carcinoma; R/M HNSCC); (ii) lung metastasis; (iii) liver metastasis. The study is being administered in the United States.
The secondary endpoints are objective response rate (ORR), safety and feasibility, and body kinetic profile.
Updated Results
Safety
NBTXR3 administration by intratumoral injection was feasible and well-tolerated. To date, the overall adverse event (AE) profile has not differed from what is expected with radiotherapy or anti-PD-1 agents. 16 serious AEs were observed, of which four (4) were identified as NBTXR3 or injection related.
Efficacy
As of the data cut-off, 16 patients in the study received NBTXR3 plus radiotherapy and 13 were evaluable for response. Tumor regression was observed in 76.9% (10/13) of evaluable patients, regardless of prior anti-PD-1 exposure. The study reported tumor regression in 80% (4/5) of anti-PD-1 naïve patients and 60% (3/5) had investigator-assessed objective response, including one (1) complete response according to response evaluation criteria outlined in RECIST 1.1. In patients with prior primary or secondary resistance to anti-PD-1, 75% (6/8) had tumor regression and 50% (4/8) had investigator-assessed objective response. These included one (1) complete response and two (2) partial responses by RECIST 1.1, along with one (1) additional investigator-assessed pathological complete response. Some patients in the study showed delayed tumor response and/or abscopal effect, suggesting NBTXR3 may potentially prime an immune response.
Spider Plot – anti-PD-1 Naïve Patients (see table 1)
Spider Plot – anti-PD-1 Refractory Patients (see table 2)
“These updated data support the potential for NBTXR3 plus radiotherapy in combination with anti-PD-1 to yield a sustained immune response in both anti-PD-1 naïve patients and patients that have progressed on prior anti-PD-1 therapy,” concluded Colette Shen, MD, PhD, an assistant professor of radiation oncology at the University of North Carolina Lineberger Comprehensive Cancer Center and Study 1100 presenting investigator at ASCO. “NBTXR3 plus radiotherapy could stimulate immune response, convert anti-PD-1 non-responders into responders, and could be a promising next step for patients who develop immune checkpoint inhibitor resistance.”
Swimmer Plot – anti-PD-1 Refractory Patients Follow-up (see table 3)
Nanobiotix Investor Event
Nanobiotix will host a virtual investor event featuring several key opinion leaders, including study investigators, after the ASCO Annual Meeting on Friday, June 11, 2021 at 8:00 am Eastern Time (14:00 Central European Time). The discussion will focus on the new immunotherapy data from Study 1100. Register here.
Gerelateerde artikelen
- 6 nieuwe doorbraken in de strijd tegen kanker worden gepresenteerd door het World Economic Forum met bijbehorende video
- 90 procent van mensen met uitgezaaide kanker heeft meerdere DNA afwijkingen. Slechts 5 procent kreeg ook optimale behandeling daarvoor.
- Antibiotica binnen een maand vooraf aan immuuntherapie met anti-PD medicijnen geeft veel slechtere resultaten op overall overleving dan zonder antibiotica bij verschillende vormen van primaire kanker.
- Anti-PD medicijnen zoals nivolumab, Pembrolizumab en atezolizumab gegeven als immuuntherapie geven zeer goede resultaten bij verschillende vormen van kanker met solide tumoren, zelfs zonder Ligand-1 receptorstatus copy 1
- Bacterien in uitzaaiingen van kankerpatienten zijn door Nederlandse onderzoekers in beeld gebracht en in een gedetailleerde catalogus opgeslagen
- Behandelen van kanker verschuift steeds meer van chemotherapie naar biologische behandelingen, gerichte therapie waaronder immuuntherapie met gemoduleerde virussen die de minste bijwerkingen geven
- Biomarkers zoals PD-L1, CD163+ en NRAS mutaties en gegevens zoals uitzaaiingen later ontstaan bepalen kans van effectiviteit van immuuntherapie met anti PD medicijnen bij melanomen
- Bloedtesten, een overzicht van recent gepubliceerde resultaten van verschillende bloedtesten
- CRISPR-Cas kan veranderingen in bacterien bewerkstelligen en via bewerkte bacterien verspreiden om zo resistentie van antibiotica te veranderen in opnieuw werkende antibiotica.
- CHRISPR-CAS9 infuus blijkt genezende behandeling voor erfelijke aandoening angio-oedeem, aldus tussenresultaten van internationale studie met Nederlandse deelname.
- CLEVER studie: Waarom kanker jaren later kan terugkeren - en hoe dit te voorkomen. CLEVER studie bij borstkankerpatienten bewijst dat een recidief is te voorkomen door tumorcellen in beenmerg te behandelen.
- De biologische processen waarom en hoe kankercellen uitzaaien wordt beter begrepen, tumorcellen vroeger ontdekt en lijkt ook steeds beter te behandelen
- De huidige staat van moleculair testen in het behandelen van kankerpatienten met solide tumoren. Een uitstekend overzichtsartikel met de nieuwste ontwikkelingen over RNA, DNA en eiwitten anno 2019
- Diagnosetest PERCEPTION via AI - Kunstmatige Intelligentie ontwikkeld en met hulp van single-cell RNA-sequencing voorspelt nauwkeurig of een specifiek medicijn van de kankerpatient zal aanslaan of resistent zal zijn.
- DRUP studie geeft bij 37 procent van de patienten alsnog een therapeutisch effect met 6 procent CR en 14 procent PR en 17 procent stabiele ziekte
- EMA: Veel nieuwe kankermedicijnen in de EU hebben geen bewezen toegevoegde waarde blijkt uit Nederlandse studie naar goedgekeurde kankermedicijnen door het Europees Geneesmiddelenbureau (EMA).
- Erfelijkheid van kanker hangt vaak af van specifieke afwijkende genencombinaties in DNA onderzoek en eiwitexpressie blijkt uit groot Whole Exome Sequencing onderzoek via de Biobank van de UK.
- ESMO - European Society for Medical Oncology heeft een gids uitgegeven voor patienten over hoe personalised medicine werkt en stand van zaken
- FDA ondersteunt onderzoek naar personalised medicine op basis van mutaties ongeacht in welk lichaamsdeel de kanker zich het eerst openbaart.
- Genetisch onderzoek via Germline testen (kiembaan testen) werd in periode 2013 tot 2019 in Georgie en Californie bij slechts 7 procent gedaan onder 1 369 602 patienten met twee jaar kanker.
- Genetische mutatie ontdekt die kans van slagen van immuuntherapie bij alle vormen van kanker naar 100 procent zou kunnen brengen
- Geneesmiddel (ARS1620) verandert kankergen (KRAS mutatie) dat kwaadaardige tumoren beschermt tegen immuunsysteem in een doelwit voor immuunsysteem en helpt immuuntherapie kankercellen te elimineren
- Gentherapie zoals Chrispr-cas en base-editors zijn zeer succesvol bij erfelijke ziekten waaronder ook vormen van kanker zoals sikkelcelziekte
- Gerichte behandelingen met Aurora kinaseremmers geven soms uitstekende resultaten bij veel vormen van kanker. Een reviewstudie
- Immuunafwijkingen bij kankerpatienten gerelateerd aan infecties voortijdig ontdekken zouden in behandelingen van kanker sterven aan kanker met 25 procent of meer kunnen voorkomen
- Immuuntherapie met HER2-gerichte CT-0508 (CAR-Macrofaag therapie) geeft bij solide tumoren van verschillende vormen van kanker met HER2 positieve expressie hoopvolle resultaten
- Immuuntherapie met pembrolizumab bij patiënten met verschillende vormen van uitgezaaide kanker met hoge microsatellietinstabiliteit (MSI-H) en DNA-mismatch-reparatie-deficiënte (dMMR) geeft uitstekende en duurzame resultaten op overall overleving
- Immuuntherapie met nivolumab zorgt voor duurzame en sterk verbeterde overall overleving bij verschillende vormen van kanker, melanomen, longkanker en nierkanker copy 1
- Interleukin-15 speelt een cruciale rol wanneer gegeven samen met immuuntherapie voor verschillende vormen van kanker met solide tumoren en biedt veelbelovende mogelijkheden voor verbeterde behandelingen
- Internationale groep van 180 wetenschappers stelt rapport op hoe en met welke niet-toxische middelen - voedingsstoffen de effectiviteit te verbeteren, recidieven te voorkomen en de bijwerkingen te verminderen van personalised medicine
- Irina Kareva gebruikt wiskundige modellen die de dynamiek van kanker beschrijven, met het doel nieuwe geneesmiddelen te ontwikkelen die gericht zijn op tumoren.
- Kanker-actueel kan en wil helpen - begeleiden bij aanvragen van een volledig biomoleculair receptorenonderzoek en genenonderzoek
- Erfelijkheid: Kanker is vaak domme pech stelt prof. dr. Nicoline Hoogerbrugge van het Radboudumc en de Radboud Universiteit in een interview in de Stentor en Algemeen Dagblad
- Kankermedicijnen geven in de klinische praktijk veel minder effect dan uit de studies van farmaceutische bedrijven is aangetoond. Maar zijn wel ontzettend duur.
- Kankerremmende eiwitten kunnen bij mutatie die gen uitschakelt veranderen van kankerremmend in stimulerend, ontdekten Nederlandse onderzoekers
- Larotrectinib geeft bijzonder goede resultaten (76 procent respons met 12 procent complete remissies) bij alle vormen van solide tumoren met een positieve TRK Fusion mutatie
- Larotrectinib: Met de goedkeuring van Larotrectinib op basis van 1 specifieke afwijking en niet op basis van primaire tumor zorgt de FDA voor een doorbraak in het behandelen van kanker
- Lenvatinib Plus Pembrolizumab bij patiënten met inoperabele gevorderde nierkanker, buikvlieskanker, melanomen en andere gevorderde kanker met solide tumoren geeft uitstekende resultaten met meer dan de helft remissies van 50 procent of meer copy 1
- Medicijnen voorschrijven op basis van DNA profiel van de patient voorkomt 30 procent minder bijwerkingen blijkt uit internationale studie onder leiding van LUMC Leiden
- Moleculaire schakelaar verandert kankercellen in weer normale cellen en zou genezende aanpak van kanker kunnen betekenen
- MSC-1 een medicijn dat de groei van de kankerstamcellen afremt door LIF blokkade en immuunsysteem activeert laat spectaculair goede resultaten zien in fase I studie.
- Mytomorrows breidt aanbod aan experimentele medicijnen voor kankerpatienten uit met 11 nieuwe nog niet geregistreerde medicijnen en stelt deze beschikbaar voor uitbehandelde kankerpatienten
- Nederland betaalt veel meer voor kankermedicijnen, soms tot 50 procent of meer, dan andere landen blijkt uit vergelijkend onderzoek tussen 18 landen copy 1
- NCI-MATCH-studie toont aan dat een biomoleculaire analyse - DNA en receptorenonderzoek - belangrijk is in hoe een kankerpatient te behandelen.
- Nieuw medicijn - PD-0332991 - stopt groei hersentumoren Glioblastoom in dierproeven. Zodra gestopt werd met dit medicijn gingen de tumoren weer groeien. Fase I studie bij 33 patienten met nierkanker en lymfklierkanker bevestigt veiligheid van dit middel
- Nieuwe, dure kankermedicijnen zijn voortaan sneller beschikbaar door het Drug Access Protocol (DAP) dat is ontwikkeld door oncologen, verzekeraars en Zorginstituut Nederland
- Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie copy 1
- Overzicht van studies met medicijnen en behandelingen om tumoren met KRAS mutaties aan te pakken. Vooral combinatiebehandelingen zijn veelbelovend.
- PI3K/AKT/mTOR pathway speelt cruciale rol in apoptose proces, DNA herstel, metabolisme in de cel en angiogenese.
- Pembrolizumab - Keytruda geeft bij solide tumoren van verschillende oorsprong 21 procent complete remissies en 53 procent gedeeltelijke remissies.
- Personalised medicine door receptorenonderzoek geeft veel betere resultaten in fase 1 studies dan experimenteel onderzoek zonder receptorenonderzoek
- Prof. Bernards over de doorbraak bij darmkanker met Kras mutatie en bij melanomen met BRAF mutatie in DWDD van donderdag 27 maart 2014
- POLE mutatie: veel kankerpatienten met erfelijke vormen van kanker hebben naast een P1-ligand een POLE mutatie en reageren goed op immuuntherapie met anti-PD medicijnen - checkpointremmers als pembrolizumab en nivolumab
- Radiotherapeutisch stimulerend middel NBTXR3 geeft in combinatie met anti-PD-1 medicijnen alsnog uitstekende resultaten bij patiënten die ziekteprogressie lieten zien ongeacht eerdere behandeling met anti-PD-1 medicijnen
- Rezatapopt, een p53-reactivator gericht op de p53 Y220C-mutatie, geeft in veiligheidstudie hoopvolle resultaten bij zwaarvoorbehandelde kankerpatienten met solide tumoren
- Rozlytrek (entrectinib), een tyrosine kinase remmer, goedgekeurd door FDA als medicijn voor solide tumoren met NTRK (neurotrophic tyrosine receptor kinase) gene fusion. Dit is 3e goedgekeurde medicijn op basis van mutatie.
- Stamceltherapie succesvol toegepast bij vrouw met diabetes type 1. Zij hoeft nu al een jaar geen insuline meer te spuiten.
- Tweede primaire vorm van kanker bij een kankerpatient wordt steeds vaker bekend bij de diagnose (2 tot 17 procent) door betere diagnose technieken en verfijnder biomoleculair onderzoek
- Tumorindeling mede aan de hand van biomarkers - biomoleculaire profielen is nodig en zal behandelingen sterk veranderen voor veel kankerpatiënten. Van 10 procent nu tot 50 procent straks. Aldus grote studie van het TOGA
- Vaccin tegen KRAS positief gemuteerde vormen van kanker - darmkankers en longkanker o.a. - wordt gecombineerd met trametinib een anti-PD medicijn in fase I studie na hoopvolle resultaten.
- Voorbeeldrapporten van receptoren en DNA testen - biomoleculaire profielen uitgevoerd door Caris Lifesciences - van alvleesklierkanker, hersentumoren, melanomen en longkanker
- Vroege diagnose van kanker is de toekomst en is vaak al mogelijk: zie TED talk
- Xeloda (capecitabine) en fluorouracil (5-FU) kunnen levensbedreigende bijwerkingen veroorzaken bij kankerpatienten met een DPD-deficiëntie. FDA pleit voor testen op genmutaties vooraf aan behandelingen.
- Ziekte van Parkinson: prasinezumab, een monoklonaal antilichaam dat alfa-synucleïne bindt, vertraagt sterk de progressie van de ziekte van Parkinson in vergelijking met patienten die beste zorg kregen
- Algemeen: overzicht van artikelen waarin personal medicine een rol speelt.



Plaats een reactie ...
Reageer op "Radiotherapeutisch stimulerend middel NBTXR3 geeft in combinatie met anti-PD-1 medicijnen alsnog uitstekende resultaten bij patiënten die ziekteprogressie lieten zien ongeacht eerdere behandeling met anti-PD-1 medicijnen"