Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
3 februari 2017: Bron: ASCO en Journal of Clinical Oncology
Op 1 februari 2017 is online een overzichtsrapport verschenen: Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology die de belangrijkste studies bespreekt die afgelopen jaar werden gepubliceerd. En voor het tweede jaar op rij wordt immuuntherapie genoemd als de belangrijkste ontwikkeling.
Vooral de anti-PD medicijn studies (checkpoint remmers) geven steeds goede hoopvolle resultaten bij nagenoeg alle vormen van kanker:
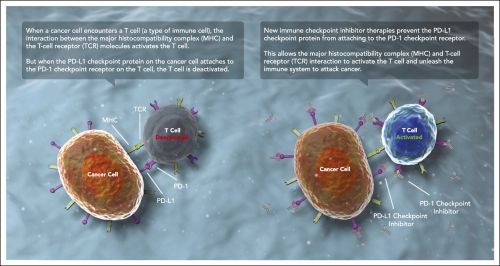
Fig 2. Immune checkpoint inhibitors: releasing the brakes on the immune system. MHC, major histocompatibility complex; PD-1, programmed death 1; PD-L1, programmed death ligand-1; TCR, T-cell receptor.
Maar ook gerichte medicijnen op bepaalde DNA mutaties en receptorenexpressie spelen grote rol in de nieuwste behandelingen van kanker. Ook preventie en screeening via ook o.a. bloedmonsters krijgen veel aandacht in dit rapport:
A range of other important advances and trends are featured in Clinical Cancer Advances:
- Precision medicine: Last year brought approvals of new treatments targeting molecules important in the growth of certain types of kidney, lung, breast, and blood cancer.
- Liquid biopsies: The first test for circulating plasma tumor DNA was approved by the FDA in 2016 for certain patients with lung cancer. This new technology allows physicians to assess key cancer-driving tumor mutations through a simple blood draw, as opposed to invasive tissue biopsies, which in turn facilitates selection of optimal treatment and monitoring changes in the status of the tumor over time.
- New tools help bridge gaps between patients and physicians: The report highlights a Web-based tool for self-monitoring symptoms that immediately alerts the cancer care team when patients report that a symptom is worsening. In addition, education and patient navigation programs demonstrate ways to increase treatment adherence.
“To conquer cancer, we must conduct research across the cancer care continuum, from screening to new treatments and strategies that help ease treatment side effects,” said Harold J. Burstein, MD, PhD, FASCO, Co-Executive Editor of Clinical Cancer Advances.
Het studierapport isd zo uitgebreid, maar ook veel van wat er in besprken wordt is ook wel op kanker-actueel te vinden dat ik maar niet verder vertaal.
Het studierapport: Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology is op verschillende manieren te bekijken. Op de website van de Journal of Clinical Oncology is het volledige rapport gratis in te zien.
Via ASCO is het rapport als flipbook in te bladeren.
Tot slot als u het studierapport wilt hebben in een PDF document kunt u ons een mailtje sturen: redactie@kanker-actueel.nl en sturen we het u digitaal toe.
Hier de inleiding tot het rapport met daaronder de referentielijst behorend bij dit studierapport:
A growing number of patients with cancer are benefiting from research advances in immunotherapy, leading ASCO to name immunotherapy as the Society's Advance of the Year for a second year in a row. Clinical Cancer Advances 2017 highlights the expanding role of immunotherapy. Evolving research findings are providing new insights on how to get optimal results from these relatively new treatments.
Source: DOI: 10.1200/JCO.2016.71.5292 Journal of Clinical Oncology - published online before print February 1, 2017
Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology
Harold J. Burstein*, Lada Krilov, Jeanny B. Aragon-Ching†, Nancy N. Baxter†, E. Gabriela Chiorean†, Warren Allen Chow†, John Frederick De Groot†, Steven Michael Devine†, Steven G. DuBois†, Wafik S. El-Deiry†, Andrew S. Epstein†, John Heymach†, Joshua Adam Jones†, Deborah K. Mayer†, Rebecca A. Miksad†, Nathan A. Pennell†, Michael S. Sabel†, Richard L. Schilsky‡, Lynn Mara Schuchter†, Nadine Tung†, Karen Marie Winkfield†, Lori J. Wirth†, and Don S. Dizon*Harold J. Burstein and Steven G. DuBois, Dana-Farber Cancer Institute; Rebecca A. Miksad and Nadine Tung, Beth Israel Deaconess Medical Center; Lori J. Wirth and Don S. Dizon, Massachusetts General Hospital, Boston, MA; Lada Krilov and Richard L. Schilsky, American Society of Clinical Oncology, Alexandria; Jeanny B. Aragon-Ching, Inova Schar Cancer Institute, Fairfax, VA; E. Gabriela Chiorean, University of Washington, Seattle, WA; Warren Allen Chow, City of Hope, Duarte, CA; John Frederick De Groot and John Heymach, University of Texas MD Anderson Cancer Center, Houston, TX; Steven Michael Devine, Ohio State University, Columbus; Nathan A. Pennell, Cleveland Clinic, Cleveland, OH; Wafik S. El-Deiry, Fox Chase Cancer Center; Joshua Adam Jones, University of Pennsylvania Health Systems; Lynn Mara Schuchter, University of Pennsylvania, Philadelphia, PA; Andrew S. Epstein, Memorial Sloan Kettering Cancer Center, New York, NY; Deborah K. Mayer, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill; Karen Marie Winkfield, Wake Forest Baptist Medical Center, Winston-Salem, NC; Michael S. Sabel, University of Michigan, Ann Arbor, MI; and Nancy N. Baxter, St Michael’s Hospital, University of Toronto, Toronto, Ontario, Canada.
I am pleased to present Clinical Cancer Advances 2017, which highlights the most promising advances in patient-oriented cancer research over the past year. The report gives us an opportunity to reflect on what an exciting time it is for cancer research and how swiftly our understanding of cancer has improved.
One year ago, the White House announced the national Cancer Moonshot program to accelerate progress against cancer. This shared vision of progress has reinvigorated the research community, identified new areas of scientific collaboration, and raised our ambitions regarding what may be possible beyond the progress we have already made.
When I entered the field 35 years ago, I could not have imagined where we would be today. We can now detect cancer earlier, target treatments more effectively, and manage adverse effects more effectively to enable patients to live better, more fulfilling lives. Today, two of three people with cancer live at least 5 years after diagnosis, up from roughly one of two in the 1970s.
This progress has resulted from decades of incremental advances that have collectively expanded our understanding of the molecular underpinnings of cancer. There is no better current example of this than ASCO’s 2017 Advance of the Year: Immunotherapy 2.0.
Over the last year, there has been a wave of new successes with immunotherapy. Research has proven this approach can be effective against a wide range of hard-to-treat advanced cancers previously considered intractable. Researchers are now working to identify biologic markers that can help increase the effectiveness of treatment and determine who is most likely to benefit from immunotherapy. This knowledge will enable oncologists to make evidence-based decisions so as many patients as possible might benefit from this new type of treatment.
Each successive advance builds on the previous hard work of generations of basic, translational, and clinical cancer researchers. Importantly, the advances described in this report would not have been possible without the individuals who volunteered to participate in clinical trials as part of their treatment.
To turn the promising vision of a cancer moonshot into meaningful advances, we need sustained, robust federal funding for continued research and innovation. Approximately 30% of the research highlighted in this report was funded, at least in part, through federal dollars appropriated to the National Institutes of Health or the National Cancer Institute. Without this federal investment—unique internationally in scale, duration, and impact for decades—I fear we may lose the forward momentum needed to further the progress we see highlighted in this report.
Federal lawmakers can further fuel progress by advancing initiatives that facilitate the use of big data to achieve the common good of high-quality care for all patients. Such programs, like ASCO’s CancerLinQ, will rapidly increase the pace of progress and dramatically expand the reach of treatment advances to the millions of patients who are living with cancer today or who will do so in the future. This investment will yield medical, scientific, economic, and societal benefits for years to come.
Much work still lies ahead. Many questions remain about how cancer develops and spreads and how best to treat it. As you read through Clinical Cancer Advances 2017, I hope you are as inspired as I am by the gains the clinical cancer research community has made over the past year and by the promise of a new era of advances just over the horizon.
Daniel F Hayes, MD, FASCO, FACP
ASCO President, 2016 to 2017
| 1. | National Cancer Institute: SEER stat fact sheets: Melanoma of the skin. https://seer.cancer.gov/statfacts/html/melan.html |
| 2. | Ribas A, Hamid O, Daud A, et al: Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315:1600-1609, 2016 CrossRef, Medline |
| 3. | Topalian SL, Sznol M, McDermott DF, et al: Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020-1030, 2014 |
| 4. | Schadendorf D, Hodi FS, Robert C, et al: Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33:1889-1894, 2015 Link |
| 5. | Postow M, Chesney J, Pavlick A, et al: Initial report of overall survival rates from a randomized phase II trial evaluating the combination of nivolumab (NIVO) and ipilimumab (IPI) in patients with advanced melanoma (MEL). Presented at the 107th Annual Meeting of the American Association for Cancer Research, New Orleans, LA, April 16-20, 2016 (abstr CT002) |
| 6. | Eggermont AM, Chiarion-Sileni V, Grob JJ, et al: Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med [epub ahead of print on October 7, 2016] |
| 7. | WHO International Agency for Research on Cancer: GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Default.aspx |
| 8. | Herbst RS, Baas P, Kim DW, et al: Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 CrossRef, Medline |
| 9. | Reck M, Rodríguez-Abreu D, Robinson AG, et al: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med [epub ahead of print on October 8, 2016] |
| 10. | Socinski M, Creelan B, Hom L, et al: CheckMate 026: A phase 3 trial of nivolumab vs investigator’s choice of platinum-based doublet chemotherapy as first-line therapy for stage IV/reccurrent programmed death ligand 1-positive NSCLC. Presented at the 2016 European Society for Medical Oncology, Copenhagen, Denmark, October 7-11, 2016 (abstr LBA7-PR) |
| 11. | US Food and Drug Administration: Atezolizumab (TECENTRIQ). http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525780.htm |
| 12. | US Food and Drug Administration: Pembrolizumab (KEYTRUDA) checkpoint inhibitor. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm526430.htm |
| 13. | National Cancer Institute: SEER stat fact sheets: Bladder cancer. https://seer.cancer.gov/statfacts/html/urinb.html |
| 14. | American Cancer Society: Bladder cancer. http://www.cancer.org/cancer/bladdercancer/ |
| 15. | US Food and Drug Administration: FDA approves new, targeted treatment for bladder cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm501762.htm |
| 16. | Rosenberg JE, Hoffman-Censits J, Powles T, et al: Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387:1909-1920, 2016 CrossRef, Medline |
| 17. | Business Wire: Merck’s KEYNOTE-045 studying KEYTRUDA® (pembrolizumab) in advanced bladder cancer (urothelial cancer) meets primary endpoint and stops early. http://www.businesswire.com/news/home/20161021005182/en/Merck%E2%80%99s-KEYNOTE-045-Studying-KEYTRUDA%C2%AE-pembrolizumab-Advanced-Bladder |
| 18. | Balar A, Bellmunt J, O’Donnell PH, et al: Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: Preliminary results from the phase 2 KEYNOTE-052 study. Presented at the 2016 European Society for Medical Oncology, Copenhagen, Denmark, October 7-11, 2016 (abstr LBA32-PR) |
| 19. | Ferlay J, Soerjomataram I, Dikshit R, et al: Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 CrossRef, Medline |
| 20. | National Cancer Institute: SEER stat fact sheets: Oral cavity and pharynx cancer. https://seer.cancer.gov/statfacts/html/oralcav.html |
| 21. | Ferris RL, Blumenschein G Jr, Fayette J, et al: Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med [epub ahead of print on October 8, 2016] |
| 22. | US Food and Drug Administration: Nivolumab for SCCHN. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm528920.htm |
| 23. | National Cancer Institute: SEER stat fact sheets: Ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html |
| 24. | Hamanishi J, Mandai M, Ikeda T, et al: Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015-4022, 2015 Link |
| 25. | National Cancer Institute: SEER stat fact sheets: Hodgkin lymphoma. https://seer.cancer.gov/statfacts/html/hodg.html |
| 26. | US Food and Drug Administration: Nivolumab (Opdivo) for Hodgkin lymphoma. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm501412.htm |
| 27. | Roemer MG, Advani RH, Ligon AH, et al: PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 34:2690-2697, 2016 Link |
| 28. | Younes A, Santoro A, Shipp M, et al: Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17:1283-1294, 2016 CrossRef, Medline |
| 29. | Armand P, Shipp MA, Ribrag V, et al: Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 34:3733-3739, 2016 |
| 30. | Zaretsky JM, Garcia-Diaz A, Shin DS, et al: Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819-829, 2016 CrossRef, Medline |
| 31. | Roberts SA, Gordenin DA: Hypermutation in human cancer genomes: Footprints and mechanisms. Nat Rev Cancer 14:786-800, 2014 CrossRef, Medline |
| 32. | Le DT, Uram JN, Wang H, et al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 CrossRef, Medline |
| 33. | Bouffet E, Larouche V, Campbell BB, et al: Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 34:2206-2211, 2016 Link |
| 34. | Nghiem PT, Bhatia S, Lipson EJ, et al: PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 374:2542-2552, 2016 CrossRef, Medline |
| 35. | Kaufman HL, Russell J, Hamid O, et al: Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 17:1374-1385, 2016 CrossRef, Medline |
| 36. | Antoniou AC, Pharoah PP, Smith P, et al: The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91:1580-1590, 2004 Medline |
| 37. | Song H, Dicks E, Ramus SJ, et al: Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol 33:2901-2907, 2015 Link |
| 38. | Holter S, Borgida A, Dodd A, et al: Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 33:3124-3129, 2015 Link |
| 38a. | Vasen H, Ibrahim I, Ponce CG, et al: Benefit of surveillance for pancreatic cancer in high-risk individuals: Outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol 34:2010-2019, 2016 |
| 39. | Yurgelun MB, Allen B, Kaldate RR, et al: Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology 149:604-613.e20, 2015 |
| 40. | Zhang J, Walsh MF, Wu G, et al: Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 373:2336-2346, 2015 CrossRef, Medline |
| 41. | Fransen M, Karahalios A, Sharma N, et al: Non-melanoma skin cancer in Australia. Med J Aust 197:565-568, 2012 CrossRef, Medline |
| 42. | Guy GP Jr, Machlin SR, Ekwueme DU, et al: Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med 48:183-187, 2015 CrossRef, Medline |
| 43. | Chen AC, Martin AJ, Choy B, et al: A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med 373:1618-1626, 2015 CrossRef, Medline |
| 44. | Zorogastua K, Erwin D, Thelemaque L, et al: Intrinsic factors of non-adherence to breast and cervical cancer screenings among Latinas. J Racial Ethn Health Disparities 3:658-666, 2016 CrossRef, Medline |
| 45. | National Cancer Institute: SEER stat fact sheets: Acute myeloid leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html |
| 46. | Stone RM, Mandrekar S, Sanford BL, et al: The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): An international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY ). Blood 126:6, 2015 |
| 47. | National Cancer Institute: SEER stat fact sheets: Acute lymphocytic leukemia (ALL). https://seer.cancer.gov/statfacts/html/alyl.html |
| 48. | Kantarjian HM, DeAngelo DJ, Stelljes M, et al: Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 375:740-753, 2016 CrossRef, Medline |
| 49. | Shaw AT, Gandhi L, Gadgeel S, et al: Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol 17:234-242, 2016 CrossRef, Medline |
| 50. | US Food and Drug Administration: FDA approves new oral therapy to treat ALK-positive lung cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm476926.htm |
| 51. | Nokihara H, Kondo M, Kim YH, et al: Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol 34, 2016 (suppl; abstr 9008) |
| 52. | National Cancer Institute: SEER stat fact sheets: Myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html |
| 53. | Palumbo A, Chanan-Khan AAA, Weisel K, et al: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study. J Clin Oncol 34, 2016 (suppl; abstr LBA4) Medline |
| 54. | Cristofanilli M, Turner NC, Bondarenko I: Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425-439, 2016 Medline |
| 55. | Finn RS, Martin M, Rugo HS, et al: PALOMA-2: Primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2– advanced breast cancer (ABC). J Clin Oncol 34, 2016 (suppl; abstr 507) |
| 56. | Hortobagyi GN, Stemmer SM, Burris HA, et al: Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738-1748, 2016 |
| 57. | US Food and Drug Administration: Palbociclib (IBRANCE capsules). http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm487080.htm |
| 58. | National Cancer Institute: SEER stat fact sheets: Kidney and renal pelvis cancer. https://seer.cancer.gov/statfacts/html/kidrp.html |
| 59. | Choueiri TK, Escudier B, Powles T, et al: Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917-927, 2016 CrossRef, Medline |
| 60. | US Food and Drug Administration: Cabozantinib (CABOMETYX). http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm497483.htm |
| 61. | Janowitz T, Welsh SJ, Zaki K, et al: Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol 40:482-491, 2013 CrossRef, Medline |
| 62. | Ravaud A, Motzer RJ, Pandha HS, et al: Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 375:2246-2254, 2016 |
| 63. | Haas NB, Manola J, Uzzo RG, et al: Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387:2008-2016, 2016 CrossRef, Medline |
| 64. | Moore KN, Martin LP, Seward SM, et al: Preliminary single agent activity of IMGN853, a folate receptor alpha (FRα)–targeting antibody-drug conjugate (ADC), in platinum-resistant epithelial ovarian cancer (EOC) patients (pts): Phase I trial. J Clin Oncol 33, 2015 (suppl; abstr 5518) |
| 65. | Buckner JC, Shaw EG, Pugh SL, et al: Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 374:1344-1355, 2016 CrossRef, Medline |
| 66. | Park JR, Kreissman SG, London SG, et al: A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study. J Clin Oncol 34, 2016 (suppl; abstr LBA3) |
| 67. | Venook AP, Niedzwiecki D, Innocenti F, et al: Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 34, 2016 (suppl; abstr 3504) |
| 68. | Tejpar S, Stintzing S, Ciardiello F, et al: Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol [epub ahead of print on October 10, 2016] |
| 69. | Petrelli F, Tomasello G, Borgonovo K, et al: Prognostic survival associated with left-sided vs right-sided colon cancer. JAMA Oncol [epub ahead of print on October 27, 2016] |
| 70. | National Cancer Institute: SEER stat fact sheets: Pancreas cancer. https://seer.cancer.gov/statfacts/html/pancreas.html |
| 71. | Neoptolemos JP, Palmer D, Ghaneh P, et al: ESPAC-4: A multicenter, international, open-label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol 34, 2016 (suppl; abstr LBA4006) |
| 72. | Lancet JE, Uy GL, Cortes JE, et al: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J Clin Oncol 34, 2016 (suppl; abstr 7000) |
| 73. | Fleshman J, Branda M, Sargent DJ, et al: Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: The ACOSOG Z6051 randomized clinical trial. JAMA 314:1346-1355, 2015 CrossRef, Medline |
| 74. | Stevenson AR, Solomon MJ, Lumley JW, et al: Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomized clinical trial. JAMA 314:1356-1363, 2015 CrossRef, Medline |
| 75. | Goss PE, Ingle JN, Pritchard KI, et al: Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375:209-219, 2016 CrossRef, Medline |
| 76. | Pan H, Gray RG, Davies C, et al: Predictors of recurrence during years 5-14 in 46,138 women with ER+ breast cancer allocated 5 years only of endocrine therapy (ET). J Clin Oncol 34, 2016 (suppl; abstr 505) |
| 77. | Navari RM, Qin R, Ruddy KJ, et al: Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375:134-142, 2016 CrossRef, Medline |
| 78. | Basch E, Deal AM, Kris MG, et al: Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 Link |
| 79. | Castaldi M, Safadjou S, Elrafei T, et al: A multidisciplinary patient navigation program improves compliance with adjuvant breast cancer therapy in a public hospital. Am J Med Qual [epub ahed of print on June 29, 2016] |
| 80. | Kaphingst KA, Blanchard M, Milam L, et al: Relationships between health literacy and genomics-related knowledge, self-efficacy, perceived importance, and communication in a medically underserved population. J Health Commun 21, 58-68, 2016 (suppl 1) CrossRef, Medline |
| 81. | National Cancer Institute: SEER stat fact sheets: Prostate cancer. https://seer.cancer.gov/statfacts/html/prost.html |
| 82. | Hamdy FC, Donovan JL, Lane JA, et al: 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375:1415-1424, 2016 CrossRef, Medline |
| 83. | Shain AH, Yeh I, Kovalyshyn I, et al: The genetic evolution of melanoma from precursor lesions. N Engl J Med 373:1926-1936, 2015 CrossRef, Medline |
| 84. | Wheler JJ, Janku F, Naing A, et al: Cancer therapy directed by comprehensive genomic profiling: A single center study. Cancer Res 76:3690-3701, 2016 CrossRef, Medline |
| 85. | Hainsworth JD, Meric-Bernstam F, Swanton C, et al: Targeted therapy for advanced solid tumors based on molecular profiles: Early results from MyPathway, an open-label, phase IIa umbrella basket study. J Clin Oncol 34, 2016 (suppl; abstr LBA11511) |
| 86. | US Food and Drug Administration: FDA approves new pill to treat certain patients with non-small cell lung cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm472525.htm |
| 87. | US Food and Drug Administration: Cobas EGFR mutation test v2. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm504540.htm |
| 88. | Jenkins S, Yang J, Ramalingam S, et al: 134O_PR: Plasma ctDNA analysis for detection of EGFR T790M mutation in patients (pts) with EGFR mutation-positive advanced non-small cell lung cancer (aNSCLC). J Thorac Oncol 11:S153-S154, 2016 (suppl) CrossRef, Medline |
| 89. | Oxnard GR, Thress KS, Alden RS, et al: Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non–small-cell lung cancer. J Clin Oncol 34:3375-3382, 2016 Link |
| 90. | Sacher AG, Paweletz C, Dahlberg SE, et al: Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol 2:1014-1022, 2016 CrossRef, Medline |
| 91. | Wakelee HA, Gadgeel SM, Goldman JW, et al: Epidermal growth factor receptor (EGFR) genotyping of matched urine, plasma and tumor tissue from non-small cell lung cancer (NSCLC) patients (pts) treated with rociletinib. J Clin Oncol 34, 2016 (suppl; abstr 9001) |
| 92. | Reckamp KL, Melnikova VO, Karlovich C, et al: A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol 11:1690-1700, 2016 CrossRef, Medline |
| 93. | Karlovich C, Goldman JW, Sun JM, et al: Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase I study of rociletinib (CO-1686). Clin Cancer Res 22:2386-2395, 2016 CrossRef, Medline |
| 94. | Zill OA, Mortimer S, Banks KC, et al: Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol 34, 2016 (suppl; abstr LBA11501) Medline |
| 95. | Tie J, Wang Y, Tomasetti C, et al: Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 8:346ra92, 2016 CrossRef, Medline |
| 96. | Mirza MR, Monk BJ, Herrstedt J, et al: Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 |
Gerelateerde artikelen
- Archief nieuwsberichten over kanker in de media
- Drie dure maar bewezen effectieve medicijnen Carvykti Ciltacabtagene Autoleucel, Enhertu Trastuzumab Deruxtecan en Xenpozyme Olipudase alfa worden niet vergoed uit het basispakket omdat ze te duur zijn
- Dertien veel voorkomende zorghandelingen in het ziekenhuis verdwijnen binnenkort uit de richtlijnen omdat de effectiviteit niet bewezen is.
- Wim Hof die wereldberoemd werd als 'de iceman' blijkt jarenlang zijn gezin geterroriseerd te hebben en wordt beschuldigd door ex-vrouw en kinderen van huiselijk geweld
- Parpremmers olaparib (Lynparza®) of niraparib (Zejula®) voor eierstokkanker en borstkanker worden gedeeltelijk uit basisverzekering gehaald door Zorginstituut Nederland
- Wetenschap is gebaseerd op een mening en te weinig kritisch, aldus Dick Veerman en collega's n.a.v. 30 diepte interviews met hoogleraren, universitaire hoofddocenten, postdocs, assistenten in opleiding (PhD’ers) en wetenschappelijke onderzoekers
- Dure medicijnen zijn oorzaak van stijgende zorgkosten. En betere opsporingstechnieken zorgt voor meer kankerdiagnoses blijkt uit onderzoek van IKNL en RIVM
- 34 procent van alle kankerpatienten stierf tussen 2010 en 2020 voor het 5e jaar na de diagnose. Toch spreekt Integraal Kankercentrum Nederland (IKNL) van een verbetering
- Kankerpatiënten met een laag inkomen hebben in Nederland een grotere kans om aan de ziekte te overlijden dan welvarende patiënten. Blijkt uit nieuw onderzoek van het Integraal Kankercentrum Nederland
- Nederlandse filmpremière “First Do No Pharm” van dr. Asheem Malhotra in Zeist op 21 november 2024
- Retour Hemel: documentaire over strijd tegen kanker van Mark Bos die zelf prostaatkanker heeft.
- Kankerpil - AOH1996, van professor Linda Malkas die alle solide tumoren zou vernietigen is aan eerste patient gegeven in fase I studie
- Patienten met zeldzame vormen van kanker overlijden vaker en krijgen latere diagnose in vergelijking met veel voorkomende vormen van kanker door het niet kunnen vinden van gespecialiseerde behandelcentra
- Sterkere MRI-scanner met nieuw contrastmiddel ontdekt uitzaaiingen van prostaatkanker in lymfklieren tot op 1 mm nauwkeurig blijkt uit Nederlandse studie aan Radboud universiteit.
- Er dreigt een wereldwijde kankerepidemie onder mensen jonger dan 50 jaar, vooral aantal darmkankerpatienten stijgt enorm blijkt uit nieuwe studie. Is er verband met corona vaccins?
- Nederlands vaccin gebruikt als immuuntherapie geneest hond van botkanker - osteacarcinoom en honden met blaaskanker reageren ook goed op het vaccin dat zich richt op het eiwit vimentine
- Je hebt kanker en hoe ga je daarmee om? Ben je streng voor jezelf, hoe ga je om met anderen die het moeilijk hebben? Kennislink interviewde 26 mensen met kanker.
- Bij meer dan 100 leerlingen van 1 school blijkt later in hun leven een zeldzame vorm van een hersentumor voor te komen. Oorzaak is nog onduidelijk.
- Frankrijk gaat groot onderzoek doen naar ontstaan en voorkomen of behandelen van endometriose met een nationaal plan van aanpak.
- Salmonella-bacterie met succes gebruikt bij muizen tegen moeilijk behandelbare kankers. Blijkt uit onderzoek aan universiteit van Leuven
- Prof. dr Michel Wouters wil met behulp van ‘real world data’ oncologische behandelprocessen verbeteren en studieresultaten toetsen aan de dagelijkse praktijk
- Tientallen ziekenhuizen schrappen dringende operaties vanwege coronadruk. Ook operaties voor kankerpatienten en stamceltransplantaties.
- Patiëntenfederatie Nederland, wordt voor meer dan 80 procent gefinancierd door het ministerie van Volksgezondheid en zorgverzekeraars en roept grote vraagtekens op over hun onafhankelijkheid.
- Veel meer longkanker in de omgeving van Tatasteel. In sommige wijken komt meer dan 50 procent meer longkanker voor in vergelijking met landelijke gemiddelde.
- UMCG Groningen gaat zelf Car-T cellen maken om zo immuuntherapie met CAR-T cellen sneller en goedkoper te geven aan kankerpatienten.
- Een nieuw ontwikkelde methode om longkankeroperaties te doen met behulp van een virtualrealitybril blijkt zeer succesvol. Aldus chirurgen uit Erasmus Medisch Centrum
- Vrouw die borstkanker overleefde maar onvruchtbaar was geworden door de chemotherapie krijgt toch baby door ingevroren eitje met sperma in te spuiten en IVF toe te passen.
- Te veel mensen, te weinig vis, dus een omega-3 (visolie) tekort. Aldus Noorse studie en gepubliceerd in Nature
- Wetenschappers ontdekken manier van immuuntherapie met T-cellen gericht op het eiwit MR1 die voor alle vormen van kanker toepasbaar zou kunnen worden.
- Diabetoloog dr. Hanno Pijl ziet de ene arts na de andere omvallen en pleit voor Nationaal gezondheidsplan om welvaartsziekten als diabetes en ook kanker te stoppen
- CAR-T celtherapie is zeer succesvol bij kankerpatienten maar loopt in Nederland vast op te strenge milieueisen, stellen 4 Nederlandse top wetenschappers
- Robert Young is een bekende Amerikaanse natuurgenezer en schreef verschillende boeken over niet toxische middelen en dieet maar kwam in de gevangenis terecht. Hier zijn verweer op video voor de Amerikaanse Commissie
- Wereldgezondheidsorganisatie (WHO) neemt traditionele Chinese geneeskunde - TCM waaronder acupunctuur op in de International Statistical Classification of Diseases and Related Health Problems (ICD).
- 300 reeds geregistreerde medicijnen voor andere ziektes - Repurposing Drugs in Oncology (ReDO) - hebben ook effect bij kanker blijkt uit onderzoek van het AntiCancer Fund
- Hoogleraar Sjaak Neefjes brengt oud kankermedicijn terug op de markt. Bekijk hier dit onderwerp in de uitzending van DWDD met Sjaak Neefjes
- Ziekenhuizen houden zich niet aan de regels voor opereren van kankerpatienten in daarvoor gespecialiseerd ziekenhuis. Honderden kankerpatienten lopen onnodig extra risico
- Nobelprijs voor Geneeskunde voor onderzoek naar cellen en zuurstof
- hormoontherapie tegen overgangsklachten geeft een hoger risico op kanker dan werd gedacht. Blijkt uit grote meta-analyse van 58 epidemologische studies
- Het is misschien toch fijner te weten dat je dood gaat. Danielle Hermans dacht ze dood ging aan longkanker maar blijft toch leven en vertelt hoe ze daar mee omgaat
- Gezonde voeding lijkt sleutel voor het indammen van chronische ziekten, zo schrijft internist Yvo Sijpkens,
- The Promise and Price of Cellular Therapies door Siddhartha Mukherjee: van stamceltransplantatie naar CAR-T celtherapie
- Relatief weinig mensen maken gebruik van medische zorg in het buitenland, ondanks dat dit ook vergoed wordt, zo meldt de Europese Rekenkamer na Europees onderzoek
- Crowdfunding voor medische doeleinden is populair maar zelden succesvol en artsen en oncologen maken zich zorgen
- Hoe betrouwbaar is de wetenschap eigenlijk? Rosanne Herzberger is daarover kritisch en Job de Vrieze bekijkt het van zijn kant in een column op Foodlog
- Red onze kinderen: stop de tabaksindustrie! Doe mee en ondersteun Anne Marie die door roken kanker kreeg en haar kinderen en alle andere kinderen daartegen wil beschermen.
- Je geld of mijn leven van de EO (Bert van Leeuwen) probeert geld op te halen voor patienten die alleen in het buitenland nog behandeld kunnen worden.
- Documentaire De zaak Tuitjenhorn over huisarts Nico Tromp en zijn vrouw Anneke en hun kinderen in conflict met Inspectie voor de Gezondheidszorg is maandag 6 mei te zien op NPO 2 om 20.25 uur.
- Tisotumab vedotin plus chemo geeft spectaculaire resultaten bij zwaarvoorbehandelde kankerpatienten met verschillende primaire vormen van kanker met solide tumoren. copy 1
- Immuuntherapie met aminozuren, MuTaTu protocol, zal binnen paar jaar alle kanker kunnen genezen schrijven Israelische onderzoekers
- De wetenschap achter de verbanning van vetzucht, alcohol en roken. Een artikel in de NRC over het preventieakkoord van Staatssecretaris Paul Blokhuis
- Hoe pembrolizumab, een nederlands immuuntherapeutisch medicijn tegen kanker, de wereld verovert
- Interview met longarts Ben van den Borne over succes van immuuntherapie bij niet-kleincellige longkanker
- Peperdure pillen, VPRO Tegenlicht volgt vijf professionals die dit niet meer accepteren en vanuit hun persoonlijke betrokkenheid in actie komen en met nieuwe modellen experimenteren. Uitzending is. zondag 7 oktober 2018 NPO 2 om 21.05 uur.]
- Anders kijken naar kanker is een serie gemaakt door Sophia van Sorgen, ex-kankerpatiente en te zien bij het Algemeen Dagblad
- Hartfalen lijkt ook verhoogd risico op darmkanker te geven. Bepaalde biomarkers (factoren) blijken voor hartfalen en darmkanker dezelfde te zijn.
- Nederlandse oncologie zit vastgeklemd in protocollen en statistieken blijkt uit studie bij kankerpatienten die voor second opinion naar buitenland gingen
- Deze 11 studies worden mogelijk gemaakt met het geld dat Maarten van der Weijden ophaalde met zijn 11 steden zwemtocht.
- Skepsis bijna blut door rechtszaak over artikel waarin zij de Italiaanse Amerikaan Ruggero Santilli beschuldigen van kwakzalverij en bedrog
- Is bacteriofaag de oplossing voor resistente bacterien waar antibiotica faalt? Antoinette Hertsenberg prijst deze aanpak aan in DWDD die al veel langer bekend is.
- Ashya King, kind met een hersentumor (medulloblastoma) is al ruim 3 jaar kankervrij door suikervrij dieet en protonenbestraling ondanks hopeloze situatie in 2014
- Voeding - Red-dieet zorgt binnen 5 weken ervoor dat 6 van de 10 kinderen van hun ADHD afkomen en geen medicijnen meer nodig hebben. BRAIN-studie zoekt nog kinderen voor deelname aan studie
- Openbaar Ministerie (OM) gaat tabaksindustrie niet vervolgen. Aanklagers - sickofsmoking - zeggen artikel 12 procedure te starten.
- Zorginstituut eist inzage in opbouw kosten van nieuwe dure medicijnen voor deze worden opgenomen in basisverzekering.
- Molucuul dat ziekte van Huntington veroorzaakt op lange termijn doodt kanker op korte termijn zonder gezonde cellen aan te tasten blijkt uit dierstudies.
- NRC publiceert twee artikelen over Wim Huppes die als arts is geschorst maar als alternatieve genezer patienten blijft behandelen met dubieuze lichttherapie
- Twee VU-hoogleraren hopen op doorbraak in de zoektocht naar een dieet dat helpt tegen depressie, adhd of autisme, aldus artikel in de Volkskrant
- Alles draait om de patient die niets te zeggen heeft. Schrijft Peter Kapitein in het Parool
- Suiker doet tumoren groeien en maakt kanker agressiever en bewijst Warburg effect. Een suikerarm dieet voor kankerpatienten is aan te bevelen aldus prof. dr. Johan M. Thevelein na zijn baanbrekende onderzoek
- Veel Nederlands voedsel (47 procent) waaronder alledaagse voedingsmiddelen zoals cornflakes, pasta en hagelslag is besmet met minerale oliën - MOAH en kunnen kanker veroorzaken.
- Prostaatkankerpatienten weten vaak niet wat het verschil is tussen behandelingsopties voor hun eigen situatie met niet uitgezaaide prostaatkanker. En realiseren zich onvoldoende wat de verschillende bijwerkingen per behandelingsoptie zijn
- Inspire2live lokt proefproces uit voor betaalbare medicijnen. Recht op gezondheid of patentrecht: wat gaat voor?
- Immuuntherapie met DNA injectie van bacterien look-a-like (CpG-B) bij beginnende melanomen geeft uitstekende resultaten in voorkomen van recidief door boost in activering van immuunsysteem
- Geen chemo of bestraling, maar zwemmen in koud water. NRC interviewde mensen die kiezen voor niet toxische aanpak, door NRC alternatieve geneeswijzen genoemd
- Alleen alternatieve behandelingen van kanker zouden risico op overlijden aan kanker hoger maken dan reguliere behandelingen.
- Nederlandse kankerpatienten overlijden na behandelingen in prive kliniek van Klaus Ross in Bracht Duitsland.
- Lymfoedeem is op te lossen met lymfkliertransplantatie, lees verhaal van ex borstkankerpatient Mirjam Bosgraaf in de Volkskrant
- Astroturf en hoe Big Pharma via de media en sociale media patienten en artsen misleidt en manipuleert. Zie de TEDtalk van Sheryll Attkisson
- KWF en NFK hebben ruzie over patienten website kanker.nl. Kankerpatienten lijken de dupe te worden van machtstrijd aldus de Volkskrant
- Thuis immuuntherapie krijgen. Anthonie van Leeuwenhoek ziekenhuis start proef met immuuntherapie voor longkankerpatienten
- Veel farmaceutische bedrijven misbruiken kankerpatienten in arme landen voor hun wetenschappelijk onderzoek, aldus kritisch rapport van de SOMO.
- Being Mum and Dad, een BBC documentaire van Rio Ferdinand, voetballer van Manchester United die zijn vrouw Rebecca verloor aan borstkanker
- Medicijnen tegen kanker innemen met ontbijt zou effectiviteit verbeteren en mindere dosering zou miljoenen kunnen besparen
- Dure medicijnen moeten goedkoper door deze zelf te maken aldus Rene Bernards en Jan Schellens in de DWDD van 8 februari 2017
- Immuuntherapie 2.0: ASCO komt met overzichtsrapport van de nieuwste ontwikkelingen bij kanker van afgelopen jaar en voorspellingen voor 2017
- Hersentumoren van type glioblastoma multiforme komt in Engeland steeds vaker voor terwijl laaggradige vormen van hersentumoren verder dalen. Is de oorzaak mobiele telefoons?
- Protonenbestraling komt beschikbaar in Delft en gaat vanaf eind 2017 volledig vergoed worden
- RIVM geeft via hun folder onjuiste en misleidende informatie over darmkankeronderzoek onder de Nederlandse bevolking.
- Bekijk aflevering van Zorg.nu over immuuntherapie met dr. Haanen en prof. dr. Schumacher d.d. 1 novermber 2016
- RTL late night uitzending met Hester Scheffer met informatie over de nanoknife - IRE - irreversible electroporation
- Farmaceutische bedrijven sponsoren en betalen artsen veel meer geld dan eerdere jaren. Onafhankelijkheid van artsen en patientenverenigingen staan op het spel
- 3-broompyruvaat, stofje waaraan patienten in kliniek van Klaus Ross overleden blijkt soms ook genezende behandeling voor uitgezaaide kanker en heeft enorme potentie
- Nederland staat tweede op wereldranglijst van aantal slokdarmkankerpatienten. Alcohol, roken, reflux - maagzuur en obesitas zijn grootste oorzaken van slokdarmkanker
- Sommige artsen helpen kankerpatienten aan nieuwe dure medicijnen door een deal met een farmaceutisch bedrijf
- De duizend-dollarpil of hoe een farmaceut de hoofdprijs vraagt voor Sovaldi een genezend medicijn voor hepatitis-C
- Urinetest en voorgeprogrammeerde probiotica kunnen kankercellen in heel vroeg stadium opsporen volgens prof. dr. Sangeeta Bhatia
- Het Farma Fortuin: Zembla woensdag 9 maart 2016. NPO 2 21.15. Over waarom medicijnen zo duur zijn en wie er allemaal aan verdienen
- Eerlijke Geneesmiddelen Voorziening (EGV) en advocatenkantoor Kienlegal spelen zeer dubieuze rol door belangenverstrengeling in vaststellen van prijzen van nieuwe dure medicijnen
- Kankermedicijnen kunnen nu getest worden op gekweekte darmtumoren, om te zien welke het beste aanslaat. Zie Hans Clevers met mooie uitleg in DWDD van 8 mei 2015
- Symposium op 21 maart 2016 over andere manier van studies opzetten met hulp van patienten voor voeding en ziektes: Beyond RCT’s: towards citizen-driven research strategies in food and health
- Nivolumab wordt slechts in 3 van de 12 ziekenhuizen gegeven aan longkankerpatienten terwijl ze verplicht zijn dit wel te geven.
- Minister Schippers wil garantie van ziektekostenverzekeraars en ziekenhuizen voor dure medicijnen bij kanker.
- Nederland betaalt veel meer voor kankermedicijnen, soms tot 50 procent of meer, dan andere landen blijkt uit vergelijkend onderzoek tussen 18 landen
- Filmmaakster Meral Uslu heeft borstkanker en vertelt daarover in haar film Mijn Kanker die wordt uitgezonden op maandag 30 november 2015 op NPO 2
- Criminelen in gevangenissen en psychiatrische patiënten krijgen voedingssupplementen binnen onderzoeksverband met als doel hun gedrag positief te beïnvloeden.
- Denny van Brenk - 33 jaar - overleeft nu 1 jaar in lever uitgezaaide galwegenkanker door chemo pomp in New York, maar hij heeft alles zelf moeten betalen
- We vertrouwen de dokter blind maar de zorg voor geen meter. Sander Heijne van de Correspondent ging uit op onderzoek maar ook hij komt er niet uit. .
- Hoe kankerpatienten hoopvolle medicijnen en behandelingen wordt onthouden waardoor zelfs oncologen in opstand komen.
- Schaken met de dood: documentaire over nieuwste medicijnen tegen kanker binnen personalised medicine in het AvL - Anthonie van Leeuwenhoek ziekenhuis.
- Alpe d'HuZes geld blijft ongebruikt voor wetenschappelijk onderzoek. Coen van Veenendaal gerehabiliteerd door Medialogica en Volkskrant
- Meeste wetenchapsgeld komt terecht bij enkele toponderzoekers. 10 procent beschikt over meer dan 60 procent van al het geld voor vrij onderzoek in Nederland.
- Het grote bedrog van de farmaceutische industrie en de toezichthouders als de EMA blootgelegd door de Correspondent in mooi stuk onderzoeksjournalistiek
- 40 procent van door FDA gevonden fraude in klinische studies wordt niet gecorrigeerd en vermeld in uiteindelijke medicijnvoorschriften.
- Patiënt moet agenda kankeronderzoek bepalen, stelt Peter Kapitein in zijn nieuwe column
- Huub Schellekens, biofarmaceut aan universiteit van Utrecht, stelt dure medicijnen aan de kaak. Radioprogramma Argos volgde hem 2 jaar. Zeer onthullende reportage



Plaats een reactie ...
Reageer op "Immuuntherapie 2.0: ASCO komt met overzichtsrapport van de nieuwste ontwikkelingen bij kanker van afgelopen jaar en voorspellingen voor 2017"